Abstract
Purpose
Allergic rhinitis (AR) is a multifactorial disease whose genetic and environmental risk factors have been studied for decades. Many pediatric studies have pointed out the familial history of allergy, hygiene hypothesis, breast-feeding, pet ownership, and diets as risk factors of AR. However, most of factors are still up for debate. This preliminary report aimed to confirm the known risk factors and find the novel risk factors for AR in the Korean pediatric population.
Methods
A bi-seasonal, winter and summer, study in 2 elementary schools included all students whose parents completed the questionnaire of medical and social histories, quality of life, infant and early-childhood history, and the living styles. Skin prick tests and endoscopic examinations were conducted on all participants.
Results
Among total 1,020 children, 338 participants had AR. The multivariate logistic regression analysis highlighted 6 factors: male gender (OR, 2.10; 95% CI, 1.32-3.33), older age (1.65; 1.03-2.65), previous history of allergic conjunctivitis (14.25; 4.99-40.74), asthma (2.73; 0.96-7.76) and pneumonia (0.39; 0.19-0.82), and an hour increase in daily playing time (0.90; 0.80-1.00).
Allergic rhinitis (AR) is a common disease in the world, especially in Western developed countries and Asian developed and developing countries. As a multifactorial disease, AR has been investigated around the world to find both genetic and environmental risk factors.1,2 Many well-organized population-based studies have been conducted and identified several risk factors.
Atopic heredity is a well-known genetically predisposed risk factor.3 Atopic heredity has further been investigated in HLA systems, polymorphism of inflammatory cytokines and single nucleotide polymorphism in eosinophil peroxidase gene.4,5,6,7 However, the environmental risk factors could be controlled to prevent the development of the disease, even with a genetic predisposition.
As a preventive modality of AR, the environmental factors have been extensively studied, but remained up for debate. Socioeconomic status (SES) has been investigated as an environmental risk factor; some studies have demonstrated a high SES as a risk factor while others showed a low SES.8,9,10 Environmental exposure to animal dander is considered to be another risk factor because of the increased frequency of sensitization and challenging.11 Indoor cat ownership was also reported as a risk factor for sensitization to cat dander.12 However, others showed that early exposure to the animal dander during the first year was associated with a lower prevalence of AR.13 More recently, it has been pointed out that the first months of life are a crucial period of inhalant allergens, particularly weed allergens.14 Diet has also been considered a risk factor for atopic diseases; fruits and vegetables provide antioxidant properties against inflammation15,16 and oily fish is a protective factor by reducing pro-inflammatory cytokines.17 However, poly-saturated fatty acid has been considered an accelerating factor by increasing cytokines.18 All of these reports are based on Western countries, which are quite different than Asian countries in terms of cultures and environments. This study aimed to investigate the risk factors in the Korean pediatric population.
The Allergic Rhinitis Cohort Study for Kids (ARCO-kids) phase I is a prospective, hospital-based study for Korean AR children from 14 medical centers since 2009. The presenting preliminary data is from ARCO-kids phase II in a community which is composed of bi-seasonal, winter and summer, check-up of AR in Korean elementary schools. The ARCO-kids phase II had started at 2012 winter as a population-based study. This preliminary report was based on the data of 2 elementary schools on December 2012 and July 2013; the schools are located in an urbanized city, Yongin, near Seoul. This study included elementary school students who and whose parents provided written informed consents. However, participants, who incompletely answered questionnaire for rhinitis symptoms, refused allergy test, or showed allergic response in negative control in allergy test, were excluded.
For the consenting participants, their parents completed self-report questionnaires. After collecting the questionnaires, endoscopic examinations of ear, nose, and throat and allergy tests were performed for all participants on 2 days at each school. The detailed protocol of this study was approved by the Institutional Review Board of Seoul National University Hospital.
The parent-reported questionnaires covered medical and social histories of participants and their parents, infant and early-childhood history, and lifestyles. Medical history included previously diagnosed diseases and nasal and ocular symptoms related to AR during the previous 12 months. The diagnosed diseases included atopic dermatitis, allergic conjunctivitis, food allergy, drug allergy, asthma, sinusitis, otitis media, pneumonia, and attention deficit hyperactivity disorder. The nasal and ocular symptoms included visual analog scales of nasal congestions, sneezing, watery or clear rhinorrhea, nasal itching, and eye discomforts including eye itching, red eye, and tearing with their duration and severity. The social history included parental smoking and drinking history during lifetime and pregnancy as well as participants' indirect smoking. The infant and early childhood history included birth weight and height, types of delivery, periods of breast and/or bottle feeding, the date starting fresh milk and weaning food, period of daycare center attendance, and scheduled vaccination. Lifestyles were evaluated based on time spent in sleep, at school, at play (indoor or outdoor activities of game or sport for fun) and in special after-school institutions for learning, sports, and arts as well as the frequency of visiting indoor and outdoor places such as gyms, swimming pools, libraries, and shopping malls.
A single ENT specialist (D.H.H.), who was blinded to participant's medical history, examined participants' ear, nose and throat using 2.7 mm 0 degree rigid endoscopes (Stryker, Kalamazoo, MI, USA), with a portable light source (UMT-511; Matrix Surgical Co., Clayton, Australia). The examination checked for otologic diseases, such as middle ear effusion, acute and chronic otitis media, nasal cavity characteristics (e.g., pale mucosa, mucosal swelling, and watery discharge), and tonsil sizes.
Skin prick tests (SPTs) were performed on the medial sides of both of participants' forearms using thirteen common standardized allergen extracts (SPT; Allergo-Pharma, Reinbek, Germany): Dermatophagoides pteronyssinus (Dp), Dermatophagoides farinae (Df), animal danders (including cat, dog; cockroach), tree pollen mixture 1 (alder, elm, hazel, popular, and willow trees), tree pollen mixture 2 (beech, birch, oak, and plane trees), grass mixture, mugwort, ragweed, oak, and molds (Alternaria alternata, and Aspergillus fumigates). Histamine (1% of histamine phosphate) and 0.9% saline were used as positive and negative control respectively. Stopping antihistamine and herbal medication are recommended three days before the SPT. Skin sensitization was defined as an average wheal diameter of any allergen greater than or equal to 3 mm.
Based on symptoms of AR from the preceding 12 months and SPT results, 4 groups (AR, non-allergic rhinitis [NAR], asymptomatic sensitization and healthy participants) were defined. A participant was diagnosed with AR if he or she had a positive SPT results and at least one of the 4 nasal symptoms, nasal congestion, sneezing, watery rhinorrhea, and nasal itching, which is not related to common cold or flu and occurs during two or more consecutive days for more than 1 hour on most days.1 A participant was diagnosed with NAR if he or she had at least one symptom without sensitization to allergens. The asymptomatic sensitization group included participants with positive SPT without any symptoms. The healthy participants group included participants without symptoms or sensitization to allergens.
Each factor from the questionnaire was evaluated with Chi-square or Fisher's exact test for categorical variables and Student t test for continuous variables. In addition, relationships were analyzed using multivariate logistic regression (Backward selection, SLS=0.1) adjusted for significant variables in the univairate analysis. SAS software package (version 9.1; SAS Institute, Inc., Cary, NC, USA) was used for the statistical analysis. Statistical significance was set at P<0.05.
Total 1,020 children included in this study with informed consents. After excluding 26 participants with incomplete questionnaire, 25 with refusal of the SPT, and 5 with saline positivity in the SPT, 964 children were finally enrolled in this study; 542 in the winter season and the other 422 in the summer season. The participants consisted of 453 males and 511 females, ranging in age from 6 to 12 years. There was no seasonal difference in positive rate of SPT; 53.8% and 50.5% positive rates in winter and summer (P=0.316). House dust mites (HDMs) are most popular positive allergen in SPT without seasonal difference; 51.0% and 46.6% in winter and summer (P=0.168). Among the participants with rhinitis symptoms, the duration and severity of the rhinitis symptoms over recent 12 months had no seasonal differences; 74.2% intermittent and 25.8% persistent symptoms in winter and 74.9% intermittent and 25.1% persistent symptoms in summer (P=0.865); 58.0% mild and 42.0% moderate to severe symptoms in winter and 53.3% mild and 46.7% moderate to severe symptoms in summer (P=0.283). The overall prevalence of AR was 35.1%; 36.9% in the winter season and 32.7% in the summer season without seasonal variations (P=0.175). Gender showed a significant difference in the prevalence: 40.4% in males and 30.3% in females (P=0.001). Children with older age (from 9 to 12 years) in elementary school had more prevalent to AR than one with younger age (from 6 to 8): 36.2% in the older and 33.1% in the younger. Among the AR participants, the highest rate of skin sensitization was found in HDMs pricks without seasonal difference; 95.5% and 93.5% in winter and summer (P=0.416). Animal dander showed marginal significance in seasonal difference of the skin sensitization; 12.5% and 20.3% in winter and summer (P=0.053). Interestingly, grass pollen showed statistically significant seasonal difference of the skin sensitization; 11.0% and 25.4% in winter and summer (P=0.001). Tree pollen, weed pollen, and fungus did not show seasonal difference; 22.5% and 23.9% for the tree pollen, 10.0% and 7.2% for the weed pollen, and 7.5% and 8.7% in fungus. Among the AR patients, symptoms duration did not show seasonal difference; 69.7% intermittent and 30.3% persistent symptoms in winter and 71.4% and 28.6% in summer (P=0.735). There were 338 participants (35.1%) were included in the AR group, 212 (22.0%) in the NAR group, 166 (17.2%) in the asymptomatic sensitization group, and 248 (25.7%) in the healthy participants group (Fig. 1).
With more than three hundreds factors, the risk factors of AR were evaluated by comparing the AR group with the healthy participants group. Using the univariate analysis, 13 risk factors were identified with significant correlations (P<0.05): male gender, older age in elementary school, previous histories of atopic dermatitis, allergic conjunctivitis, food allergy, asthma, and otitis media, the first child in the family, decreased daily playing time, frequent visits to an indoor gyms, indoor playgrounds, and shopping malls, and paternal or maternal allergic rhinitis (Table 1). There were 3 additional risk factors with a marginal significance (P<0.10); previous history of pneumonia and paternal and maternal drinking histories (Table 1).
The multivariate analysis was performed with the above 16 risk factors. It produced 4 risk factors that were statistically significantly correlated with AR (P<0.05); male gender, older age, previous history of allergic conjunctivitis and pneumonia (Table 2). There were also 2 additional risk factors with a marginal significance (P<0.10); previous history of asthma and decreased daily playing time (Table 2).
Among total 129 participants reported previous pneumonia history, there was 36 participants in the AR group and 41 in the healthy participants group; average onset age 3.5 years old in the AR and 3.2 years old in the healthy participants (P=0.590). Only one participant in the AR group had a pneumonia during infancy while 2 in the healthy participants.
The six risk factors from the multivariate analysis were evaluated in the 4 groups. Except for the older age and pneumonia history, the other 4 factors were more highly related to the AR group than the NAR and asymptomatic sensitization groups (Fig. 2). The male gender and previous history of allergic conjunctivitis and asthma were most common 54.1%, 29.0%, and 11.6% in the AR group followed by the NAR and asymptomatic sensitization groups (Fig. 2A, B, and C). The daily playing time was the lowest 2.56 hours in the AR group followed by the asymptomatic sensitization and NAR groups (Fig. 2D). The older age in the elementary school took largest proportion in the asymptomatic sensitization group followed by the AR group (Fig. 2E). Previous history of pneumonia was highest 17.1% in the healthy participants group; the other groups had similar lower pneumonia histories (Fig. 2F).
The results of the physical and endoscopic examinations were investigated to find differences between the AR and the healthy participants groups (Table 3). The age-adjusted percentile of body mass index showed no difference (P=0.942). Overweight and obese participants did not have a greater risk of AR than others.
The endoscopic examinations indicated that pale mucosa, mucosal swelling, and/or watery discharge were highly related to AR (P<0.001) while purulent or mucoid discharge, posterior nasal drip, and tonsillar hypertrophy were not (P=0.484, P=0.961, P=0.658, and P=0.504). A multivariate analysis was conducted for the pale mucosa and watery discharge with gender and age adjustment; there was no participant in the healthy participants group with mucosal swelling. It showed that both pale mucosa and watery discharge had a marked OR 4.472 (95% CI, 2.295-8.713) with P<0.001 and 2.766 (1.322-5.784) with P=0.007.
Following the results of other studies, our study confirmed that the male gender and parental history of AR are risk factors of AR.19,20 Male predominance would be resolved with growing age until eventually there is no gender predominance of AR in the adult population.19 However, our study did not show the increase of female AR in more advanced ages because of participants' age range (elementary school students are limited to younger than 13 years old). Parental AR is a well-known genetic risk factor. The association between atopic family history and sensitization was shown to be strong with OR, from 1.8 to 3.0.20 Our study indicated that children with a parental AR had higher prevalence in AR group.
Other studies had already noticed increasing prevalence of pediatric AR with aging and some of them reported second decade of life as peak age of prevalence.21 The similar result was replicated in our study; the older age, from 9 to 12 years, in elementary school was pointed out as a risk factor of AR in the elementary school. It might be caused by the increased chance of exposure to allergens from the environment as moving up to senior grades in elementary school.
Our study showed the considerably higher sensitization rates to HDMs in Korean children with AR.22 The frequent sensitization and challenging of the HDM allergen would take place in the indoor gyms, indoor playgrounds and shopping malls, where the concentration of HDM would increase more than the outdoors. That explains why the AR group tended to visit frequently the indoor places. Effective control of HDMs in the indoor places might be preventing development or symptoms of AR.
This study found 2 novel risk factors for AR among Korean pediatric population: lack of pneumonia history and short playing time during the day. For decades, the hygiene hypothesis has been upheld as an important environmental factor inducing allergic diseases. The hypothesis is based on the early period viral infection preventing the development of allergic diseases.23,24,25 Since pneumonia is sequelae of severe and/or frequent respiratory infections, pneumonia history might give children protective effect to AR by the hygiene hypothesis. In the multivariate analysis, our study showed about 60% reduction of AR by pneumonia history. The lower airway infection showed difference effects on the AR depending on the severity and onset age. However, our study did not show any difference in the onset age of pneumonia between participants in the AR and healthy participants group. Beside the AR group, the NAR and asymptomatic sensitization groups had lower rates of pneumonia history than the healthy participants group; the rates were 11.4% similar to the rate of the AR group. Since the onset age was the only surveyed item for the pneumonia, our study has a limitation to prove the whether there were common characteristics of the three groups different from the healthy participants group or not. Further study would survey more detailed history of pneumonia, such as symptom duration, administration and antibiotics medication periods, and the numbers of pneumonia. The family size and number of siblings, which are classical risk factors in the hygiene hypothesis, were insignificant in our study.
The playing time is the most enjoyable and the least stressful time during the day. Some studies have pointed out that emotionally stressful situation might trigger atopic diseases. It has been reported that maternal stressful life events during pregnancy increased the risk of atopic diseases.26 The attenuated cortisol response to stress has also been published.27 The ARIA group also described stress as one of the factors influencing the prevalence of AR.1 However, to prove the relationship between AR development and emotional stress, further study is needed with more precise questions reflecting the quantity and quality of emotional stress and its accumulated amount. However, no question to discriminate playing time into indoor and outdoor time in survey is one of the limitations of our study.
As the preliminary reports, our study has limitations in terms of the area and size of the survey; ARCO-kids phase II in a community did not cover the rural pediatric population, resulting in an inability to evaluate different levels of susceptibility to AR between urban and rural individuals. However, our study has great merit in terms of the individual endoscopic examinations. Few population-based studies have produced such valuable endoscopic findings from the pediatric population. Our study may provide the first evidence of known nasal endoscopic findings in AR. In particular, pale mucosa and watery discharge were observed 4.5 and 2.8 times more frequently in the AR group than in the healthy participants group. The bi-seasonal design of the study also provided several important results, no seasonal difference in the prevalence of Korean pediatric AR, the skin sensitization rate in major allergen HDM and symptom duration and severity in the AR group.
In conclusion, this preliminary report population showed somewhat different from that of Western countries. HDM is the major allergen of Korean pediatric AR and children with AR are apt to visit the indoor places more frequently. In particular, lack of pneumonia history and short playing time are newly found risk factors to the Korean pediatric AR. Furthermore, our study might be the first epidemiologic study showing the nasal endoscopic findings and their correlation with AR.
Figures and Tables
Fig. 1
Distribution of allergic and non-allergic rhinitis, asymptomatic sensitization and healthy participants in the ARCO-Kids phase II in a community, 2012-2013.
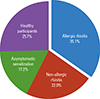
Fig. 2
Proportion and average of the 6 risk factors in AR, NAR, asymptomatic sensitization and healthy participants groups; (A) male gender, previous histories of (B) allergic conjunctivitis and (C) asthma, (D) daily playing time, (E) older age, and (F) previous history of pneumonia. AR, allergic rhinitis; NAR, non-allergic rhinitis.

Table 1
Selected characteristics of allergic rhinitis and healthy participants in the ARCO-Kids phase II in a community, 2012-2013

Table 2
Risk factors for allergic rhinitis in the ARCO-Kids phase II in a community, 2012-2013

*Multivariate logistic regression (Backward selection, SLS=0.1) adjusted for significant variables shown in Table 1.
ARCO-kids, allergic rhinitis cohort study for kids.
Table 3
Age-adjusted percentile of BMI and endoscopic findings in nasal and oral cavity

ACKNOWLEDGMENTS
The authors would like to thank Hyo Jin Yim and ARCO-kids team for their assistance for survey and skin prick tests. This work was partly supported by the Korean Center for Disease Control and Prevention (grant number, 2012-E33003-00).
References
1. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 63:Suppl 86. 8–160.
2. Portelli MA, Hodge E, Sayers I. Genetic risk factors for the development of allergic disease identified by genome wide association. Clin Exp Allergy. 2014.
3. Dold S, Wjst M, von Mutius E, Reitmeir P, Stiepel E. Genetic risk for asthma, allergic rhinitis, and atopic dermatitis. Arch Dis Child. 1992; 67:1018–1022.
4. Barnes KC, Marsh DG. The genetics and complexity of allergy and asthma. Immunol Today. 1998; 19:325–332.
5. Joki-Erkkilä VP, Karjalainen J, Hulkkonen J, Pessi T, Nieminen MM, Aromaa A, et al. Allergic rhinitis and polymorphisms of the interleukin 1 gene complex. Ann Allergy Asthma Immunol. 2003; 91:275–279.
6. Nieters A, Linseisen J, Becker N. Association of polymorphisms in Th1, Th2 cytokine genes with hayfever and atopy in a subsample of EPIC-Heidelberg. Clin Exp Allergy. 2004; 34:346–353.
7. Nakamura H, Higashikawa F, Miyagawa K, Nobukuni Y, Endo T, Imai T, et al. Association of single nucleotide polymorphisms in the eosinophil peroxidase gene with Japanese cedar pollinosis. Int Arch Allergy Immunol. 2004; 135:40–43.
8. Mercer MJ, Joubert G, Ehrlich RI, Nelson H, Poyser MA, Puterman A, et al. Socioeconomic status and prevalence of allergic rhinitis and atopic eczema symptoms in young adolescents. Pediatr Allergy Immunol. 2004; 15:234–241.
9. Almqvist C, Pershagen G, Wickman M. Low socioeconomic status as a risk factor for asthma, rhinitis and sensitization at 4 years in a birth cohort. Clin Exp Allergy. 2005; 35:612–618.
10. Durkin MS, Islam S, Hasan ZM, Zaman SS. Measures of socioeconomic status for child health research: comparative results from Bangladesh and Pakistan. Soc Sci Med. 1994; 38:1289–1297.
11. Park YB, Mo EK, Lee JY, Kim JH, Kim CH, Hyun IG, et al. Association between pet ownership and the sensitization to pet allergens in adults with various allergic diseases. Allergy Asthma Immunol Res. 2013; 5:295–300.
12. Roost HP, Künzli N, Schindler C, Jarvis D, Chinn S, Perruchoud AP, et al. Role of current and childhood exposure to cat and atopic sensitization. European Community Respiratory Health Survey. J Allergy Clin Immunol. 1999; 104:941–947.
13. Hesselmar B, Aberg N, Aberg B, Eriksson B, Björkstén B. Does early exposure to cat or dog protect against later allergy development? Clin Exp Allergy. 1999; 29:611–617.
14. Zhumambayeva S, Rozenson R, Tawfik A, Awadalla NJ, Zhumambayeva R. Date of birth and hay fever risk in children and adolescents of Kazakhstan. Int J Pediatr Otorhinolaryngol. 2014; 78:214–217.
15. Seaton A, Godden DJ, Brown K. Increase in asthma: a more toxic environment or a more susceptible population? Thorax. 1994; 49:171–174.
16. Bodner C, Godden D, Brown K, Little J, Ross S, Seaton A. Aberdeen WHEASE Study Group. Antioxidant intake and adult-onset wheeze: a case-control study. Eur Respir J. 1999; 13:22–30.
17. Hodge L, Salome CM, Peat JK, Haby MM, Xuan W, Woolcock AJ. Consumption of oily fish and childhood asthma risk. Med J Aust. 1996; 164:137–140.
18. Black PN, Sharpe S. Dietary fat and asthma: is there a connection? Eur Respir J. 1997; 10:6–12.
19. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001; 108:S2–S8.
20. Wahn U, Lau S, Bergmann R, Kulig M, Forster J, Bergmann K, et al. Indoor allergen exposure is a risk factor for sensitization during the first three years of life. J Allergy Clin Immunol. 1997; 99:763–769.
21. Sibbald B. Epidemiology of allergic rhinitis. Monogr Allergy. 1993; 31:61–79.
22. Lee JE, Ahn JC, Han DH, Kim DY, Kim JW, Cho SH, et al. Variability of offending allergens of allergic rhinitis according to age: optimization of skin prick test allergens. Allergy Asthma Immunol Res. 2014; 6:47–54.
23. Shaheen SO, Aaby P, Hall AJ, Barker DJ, Heyes CB, Shiell AW, et al. Measles and atopy in Guinea-Bissau. Lancet. 1996; 347:1792–1796.
24. Lewis SA, Britton JR. Measles infection, measles vaccination and the effect of birth order in the aetiology of hay fever. Clin Exp Allergy. 1998; 28:1493–1500.
25. Bodner C, Godden D, Seaton A. The Aberdeen WHEASE Group. Family size, childhood infections and atopic diseases. Thorax. 1998; 53:28–32.
26. de Marco R, Pesce G, Girardi P, Marchetti P, Rava M, Ricci P, et al. Foetal exposure to maternal stressful events increases the risk of having asthma and atopic diseases in childhood. Pediatr Allergy Immunol. 2012; 23:724–729.
27. Wamboldt MZ, Laudenslager M, Wamboldt FS, Kelsay K, Hewitt J. Adolescents with atopic disorders have an attenuated cortisol response to laboratory stress. J Allergy Clin Immunol. 2003; 111:509–514.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download