Abstract
Purpose
This study investigated the long-term efficacy, safety, and compliance associated with sublingual immunotherapy (SLIT) in Korean patients with allergic rhinitis sensitized to house dust mites.
Methods
This is a retrospective cohort study. A total of 164 patients who were sensitized to Dermatophagoides pteronyssinus and Dermatophagoides farinae and who received SLIT were enrolled between November 2007 and January 2010. Each patient was followed up using a diary card, on which a symptom score, rescue medication score, and adverse events (AEs) were recorded.
Results
All allergic rhinitis symptoms improved after 3 years of SLIT (P<0.05), and the rescue medication score decreased with time (P<0.05). The incidence of AEs associated with SLIT was 31% (51 of 164 patients) during the first month of therapy, and there were no severe AEs. The dropout rate was 19.5% (32 of 164 patients) during the first month, 34% (56 of 164 patients) after 6 months, and 41% (68 of 164 patients) after 1 year of SLIT. The 3-year compliance rate was approximately 40% (65 of 164 patients). The most common causes of dropout during the first month of SLIT were high cost and inconvenience. The improvement in allergic symptoms was the most common cause of dropout after 6 months.
Conclusions
Allergic symptoms significantly decreased after 1 year of SLIT treatment, and this effect was sustained after 2 or 3 years of treatment. By increasing compliance through patient education, the 3-year use of SLIT for house dust mite allergies may be effective in the management of allergic rhinitis.
Specific immunotherapy has been used for many years worldwide. The use of sublingual immunotherapy (SLIT) has increased in Europe over the past 20 years.1 However, in the United States, subcutaneous immunotherapy (SCIT) has been the traditional form of immunotherapy for inhalant allergies, and it is currently the only immunotherapy method approved by the U.S. Food and Drug Administration.2 SLIT has been gradually accepted as a viable alternative to SCIT.3 According to the World Health Organization (WHO) Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines, SLIT may be effective for pollen or mite allergic rhinitis (AR).4 A recent meta-analysis has reported the efficacy of SLIT for house dust mites (HDMs).5 This study has reported significant reductions in the symptoms and amount of rescue medications used in the SLIT group compared with the placebo group.
SCIT and SLIT are safe and effective treatments for AR, but high levels of compliance and persistence are crucial to achieve desired clinical effects. Real-life treatment adherence is better in SCIT recipients than in SLIT recipients, although it is low overall. There is an urgent need for further identification of potential barriers and measures that will enhance persistence and compliance.6 SLIT has been recently introduced in Korea, where it is more frequently used than SCIT. We have reported the short-term (6 months and 1 year) efficacy and safety of SLIT as well as early compliance with this therapy.7,8 However, long-term results (>1 year) have not yet been published in Korea. Thus, this study was performed to investigate efficacy, adverse events (AEs), and long-term compliance following 3 years of SLIT in Korean patients with AR sensitized to HDMs.
Patients who were diagnosed with AR and sensitized to Dermatophagoides pteronyssinus (Dp) and Dermatophagoides farinae (Df) at Seoul National University Hospital were indicated for SLIT. Patients received this therapy from November 2007 to January 2010 and were scheduled to undergo 3 years of SLIT. Patients with asthma or atopic dermatitis who did not require the regular use of medications were enrolled; those with symptomatic asthma or atopic dermatitis who required regular medications, such as oral or inhaled steroids or antihistamines, were excluded. Pregnant or lactating women, as well as subjects who suffered from immunological or hematological disorders, were also excluded.
Table 1 shows the patient demographic data on gender, age, severity of allergic symptoms (ARIA guidelines), mean allergic symptom duration, and the proportions of patients with atopic dermatitis and asthma, and family history of AR. Sensitization to Dp and Df was defined as (1) a serum-specific IgE level of ≥0.7 UI/mL for Dp and Df as shown by multiple allergen simultaneous tests (MASTs) or (2) wheal diameters for Dp and Df equal to or greater than those of the positive control (histamine) on skin prick tests.9
Informed consent was obtained from all patients or their parents. The Institutional Review Board of the Clinical Research Institute at Seoul National University Hospital approved the study protocol (IRB No. 1310-109-530).
A standardized HDM extract (50% Dp/50% Df, Pangramin SLIT®, ALK-Abello, Madrid, Spain) was used for immunotherapy. During a 4-week up-dosing phase, the participants were administered daily increasing doses as follows: 1 to 5 drops of a 1.6 STU/mL solution from days 1 to 10, 1 to 5 drops of an 8 STU/mL solution from days 11 to 15, 1 to 5 drops of a 40 STU/mL solution from days 16 to 20, 1 to 5 drops of a 200 STU/mL solution from days 21 to 25, and 1 to 5 drops of a 1,000 STU/mL solution from days 26 to 30. After reaching the maintenance dose (5 drops of 1,000 STU/mL solution), the participants were administered the allergen 3 times per week during the maintenance phase. The patients were told to hold the drops of allergen under their tongue for 2-3 minutes before swallowing.
All patients were asked to complete questionnaires before receiving SLIT and at 6 months, 1 year, 2 years, and 3 years after receiving this therapy, during which time no medications were taken. The questionnaire included the following symptoms: rhinorrhea, sneezing, nasal obstruction, itchy nose, and eye discomfort. Each symptom was graded from 0 to 5 (0: no symptom; 1: very mild symptom; 2: mild; 3: moderate; 4: severe; 5: very severe). The total symptom score (TSS) was defined as the sum of the scores of the 5 symptoms.
When the symptoms of AR were aggravated during immunotherapy, the patients were allowed to use antihistamines and/or intranasal steroids. They were also asked to assess their medication use. The rescue medication score (RMS) was defined as the sum of monthly medication use (1 point for an antihistamine tablet, intranasal antihistamine, or ocular antihistamine; 2 points for an intranasal corticosteroid) according to the statement of the World Allergy Organization task force.10
The patients were classified into 2 groups depending on whether they did (effective response group) or did not (ineffective response group) possess at least a 30% TSS reduction compared to their baseline score. This criterion was modified from a previous study,8 which evaluated allergen-specific immunotherapy as effective if the mean of the visual analog scale scores showed at least a 30% decrease with respect to the visual analog scale score before treatment. The effective response ratio (ER ratio) was defined as the number of patients in the effective response group divided by the total number of patients evaluated during the study period.
AEs included any untoward medical occurrences experienced by a patient administered SLIT that did not necessarily have a causal relationship with the therapy. The participants recorded SLIT-related AEs on a daily basis using diary cards throughout the treatment period. To evaluate the severity of the AEs, they were classified according to the grading of systemic reactions to immunotherapy as reported by the European Academy of Allergology and Clinical Immunology (EAACI) (Table 2).11 To evaluate the type of AE, all patients reported their symptoms, such as oral itching and swelling, gastrointestinal problems, aggravation of AR symptoms, and systemic problems, throughout the treatment period.
Compliance was defined as patient adherence to a recommended course of treatment. We considered the dropout rate when calculating compliance. Long-term compliance was defined as treatment lasting for more than 3 years. Participants who discontinued SLIT were interviewed via telephone to ascertain the reason for their cessation.
The symptoms and rescue medication score before and after SLIT were statistically analyzed via the paired t and Wilcoxon signed rank tests. SPSS (Version 17.0; SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses. All of the tests were 2-tailed, and statistical significance was set at P<0.05.
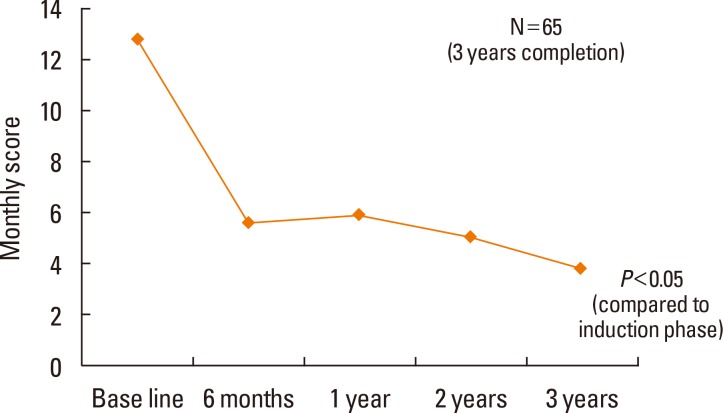
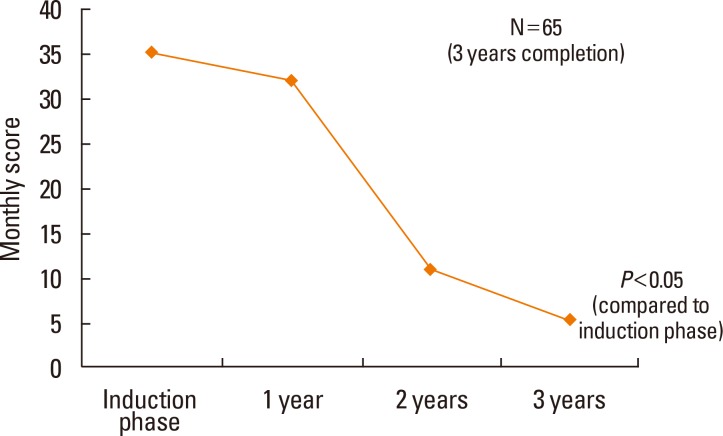
Both the TSS and RMS significantly decreased as a result of SLIT treatment (P<0.05; Fig. 1 and 2, respectively). Among 132 patients, 104 (79%) belonged to the effective response group after 6 months of SLIT. Among 65 patients, 47 (72%) belonged to the effective response group after 3 years (Table 3). The ER ratios were 0.79 after 6 months, 0.69 after 1 year, 0.56 after 2 years, and 0.72 after 3 years.
Of 164 patients, 94 reported no AEs during the first month of SLIT, 40 experienced grade 1 AEs, 23 had grade 2 AEs, and 7 possessed grade 3 AEs according to the EAACI grading system. These AEs were temporary and spontaneously subsided without medications. None of the patients required an emergency department visit due to their AEs. Grade 4 and 5 AEs were not reported during the SLIT study period (Table 4).
In addition, 57 of 108 patients reported AEs after 6 months; 53 of 96 patients experienced AEs after 1 year; 22 of 65 patients reported AEs after 2 years of SLIT. The incidence of SLIT AEs over the entire study period was 43% (70 of 164 patients). Overall, 16 patients reported oral itching, and 5 patients reported oral mucosal swelling. Nineteen patients had gastrointestinal symptoms, and 51 and 26 patients experienced transient systemic problems, such as dyspnea and asthma, respectively (Table 5).
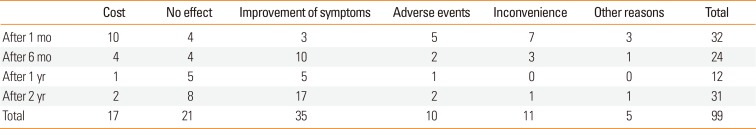
Of 164 patients, 32 dropped out after 1 month, 24 dropped out after 6 months, 12 dropped out after 1 year, 31 dropped out after 2 years, and 65 completed the 3-year treatment schedule. The 3-year long-term compliance rate was 40% (65 out of 164 patients). The reasons for patient dropout during the first month of SLIT were primarily high cost and inconvenience, and after 6 months of this therapy, additional patients dropped out due to the improvement in their allergic symptoms or perceived ineffectiveness (Table 6).
Over the entire study period, the most common cause of dropout was the improvement in allergic symptoms (n=35). AEs causing dropout included the aggravation of allergic symptoms (n=6), fever (n=2), gastrointestinal problems (n=3), eye swelling (n=2), and skin rash (n=3). Other reasons for dropout included residential relocation (n=5), pregnancy (n=3), business (n=2), and emigration (n=1).
SLIT has received significant attention as a valid treatment for AR. Similar to our previous study,7,8 this study also revealed that allergic symptoms were significantly decreased after 1 year of SLIT treatment and continued to decline after 2 or 3 years of treatment. Additionally, the use of rescue medications significantly decreased with SLIT treatment. This study showed a 30% reduction in TSS in 71% of the 3-year SLIT treatment patients. The ER ratio was 0.79 after 6 months, which decreased to 0.56 after 2 years but increased to 0.72 after 3 years.
A meta-analysis of 15 randomized, double-blind, placebo-controlled SLIT trials involving 257 patients with HDM-induced rhinitis has been published.12 Two trials in the meta-analysis showed significant differences between the active treatment and placebo groups as measured by a symptom score or a symptom-related efficacy criterion. One study found a significant difference in favor of active treatment as measured by the area under the curve of the total nasal symptom score (P<0.03) after 12 months of administration of a maintenance dose of 9 mg of Der p 1.13 A 24-month study using carbamylated allergoid tablets of an undefined dose revealed a significant difference between the active treatment and placebo groups in the total symptom score (without quoting values) for the first year of the study (P=0.027), but not the second.14 They observed the same pattern for the medication score. Compared to the pharmacotherapy group, the total rhinitis symptom score, total asthma symptom score, total medication score, and VAS score were significantly reduced in the SLIT group after 12 months of treatment.15
The safety of SLIT has been confirmed by many trials. In our study, 70 (43%) of 164 patients experienced AEs, the most common of which was the aggravation of AR symptoms. The patients tended to report the symptomatic aggravation of AR as an AE, but this phenomenon was not a serious AE because it involved both a true AE and disease aggravation due to environmental allergens.
Some studies of the SLIT treatment of patients from Western countries have reported drop-out rates. In a 2-year study, 154 of 204 youth (aged 6-18 years) receiving grass-pollen extract completed the study; thus, the dropout rate was 23%. The main reason for discontinuation was the inability to take medication according to schedule.16 In another study, 226 of 443 adult or adolescent patients received once-daily SLIT (SLITOne®) for 6 months. The data on compliance were obtained in the 3rd month for all 443 patients and in the 6th month for 266 patients because the remaining 217 subjects had received a preseasonal treatment that lasted only 3 to 4 months. Thus, compliance in the 6th month was 49%. Thirty patients discontinued their SLIT regimen for mites at 6 months, and the most common reason was side effects that were not related or possibly related to SLIT (33%, 10 of 30 patients).17
In our study, the dropout rate for patients receiving SLIT after 3 years was 60% (99 of 164 patients), and the 3-year long-term compliance rate was 40% (65 of 164 patients). The reasons for dropout included high cost, inconvenience, and AEs experienced during the first month of SLIT, which changed to improvements in allergic symptoms and the ineffectiveness of SLIT after 6 months. Of the 99 patients who dropped out, 35 did so due to symptom improvement, which should be taken into consideration when evaluating the 60% dropout rate. After 2 years, the dropout rate due to symptom improvement was 2-fold higher than that due to treatment ineffectiveness; most of the patients who dropped out due to treatment ineffectiveness did so before 2 years of treatment, while those who continued SLIT primarily reported receiving effective treatment.
The reasons for discontinuing SLIT differed between Korea and Western countries, which may have been due in part to differences in the cost of treatment. The cost of SLIT for HDMs is currently $100 per month in the USA18 and $150 per month in Korea, the latter of which is a significant burden for Korean patients. Of the 99 patients who dropped out, 11 did so due to inconvenience. In 2013, we reported that the administration of once-daily SLIT without dose escalation was welltolerated and showed the levels of safety and efficacy comparable to those achieved using a conventional escalation regimen.19 It may be possible to obtain higher compliance with a once-daily SLIT regimen compared to a conventional escalation regimen.
A study of 137 patients with AR due to HDM has suggested that a treatment duration of 3 years ensures substantial long-term effects.17 Another study performed on 186 patients with seasonal AR revealed that although no significant differences were found between the actively treated and placebo groups after 1 year of SLIT, there was a significant symptom reduction after the second year of therapy.20 However, a recent report documented that 100 (49.3%) of 203 AR patients who underwent 4 years of SLIT showed inadequate clinical responses.21 Additional long-term studies are required to determine the duration of the reduction in allergic symptoms after SLIT to provide information on appropriate timetables for its application.
One limitation of this study was the lack of control subjects, such as patients treated with standard medications or placebos. Thus, it is difficult to evaluate the relative efficacy of SLIT compared to that of standard medications or placebos in the treatment of AR. Another limitation was that the follow-up period following SLIT treatment was not long. Follow-up monitoring will be necessary to confirm that the effects of SLIT persist after 3 years. The efficacy, safety, and low compliance of a 3-year SLIT treatment were demonstrated in this study. Given that the reasons for early dropout mainly involved high cost and inconvenience, reducing costs, simplifying the up-dosing schedule, and patient education may allow for improved compliance. Most dropouts after 6 months occurred when the patients perceived improvements in their allergic symptoms; thus, clinicians should encourage patients to continue SLIT for at least 3 years in order to maintain the desired effects of this therapy.
References
1. Saporta D. Sublingual immunotherapy: a novel, albeit not so new, immunotherapy treatment modality. Am J Rhinol. 2008; 22:253–257. PMID: 18211743.

2. Wise SK, Schlosser RJ. Subcutaneous and sublingual immunotherapy for allergic rhinitis: what is the evidence? Am J Rhinol Allergy. 2012; 26:18–22. PMID: 22391071.

3. Bahceciler NN, Cobanoglu N. Subcutaneous versus sublingual immunotherapy for allergic rhinitis and/or asthma. Immunotherapy. 2011; 3:747–756. PMID: 21668312.

4. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 63(Suppl 86):8–160. PMID: 18331513.
5. Compalati E, Passalacqua G, Bonini M, Canonica GW. The efficacy of sublingual immunotherapy for house dust mites respiratory allergy: results of a GA2LEN meta-analysis. Allergy. 2009; 64:1570–1579. PMID: 19796205.
6. Kiel MA, Röder E, Gerth van Wijk R, Al MJ, Hop WC, Rutten-van Mölken MP. Real-life compliance and persistence among users of subcutaneous and sublingual allergen immunotherapy. J Allergy Clin Immunol. 2013; 132:353–360.e2. PMID: 23651609.

7. Chang H, Han DH, Mo JH, Kim JW, Kim DY, Lee CH, et al. Early compliance and efficacy of sublingual immunotherapy in patients with allergic rhinitis for house dust mites. Clin Exp Otorhinolaryngol. 2009; 2:136–140. PMID: 19784406.

8. Kim ST, Han DH, Moon IJ, Lee CH, Min YG, Rhee CS. Clinical and immunologic effects of sublingual immunotherapy on patients with allergic rhinitis to house-dust mites: 1-year follow-up results. Am J Rhinol Allergy. 2010; 24:271–275. PMID: 20819465.

9. Milián E, Díaz AM. Allergy to house dust mites and asthma. P R Health Sci J. 2004; 23:47–57. PMID: 15125219.
10. Canonica GW, Baena-Cagnani CE, Bousquet J, Bousquet PJ, Lockey RF, Malling HJ, et al. Recommendations for standardization of clinical trials with Allergen Specific Immunotherapy for respiratory allergy. A statement of a World Allergy Organization (WAO) taskforce. Allergy. 2007; 62:317–324. PMID: 17298350.

11. Canonica GW, Bousquet J, Casale T, Lockey RF, Baena-Cagnani CE, Pawankar R, et al. Sub-lingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy. 2009; 64(Suppl 91):1–59. PMID: 20041860.
12. Calderon MA, Casale TB, Nelson HS, Demoly P. An evidence-based analysis of house dust mite allergen immunotherapy: a call for more rigorous clinical studies. J Allergy Clin Immunol. 2013; 132:1322–1336. PMID: 24139829.
13. Tonnel AB, Scherpereel A, Douay B, Mellin B, Leprince D, Goldstein N, et al. Allergic rhinitis due to house dust mites: evaluation of the efficacy of specific sublingual immunotherapy. Allergy. 2004; 59:491–497. PMID: 15080829.

14. Pauli G, Bessot JC, Bigot H, Delaume G, Hordle DA, Hirth C, et al. Clinical and immunologic evaluation of tyrosine-adsorbed Dermatophagoides pteronyssinus extract: a double-blind placebo-controlled trial. J Allergy Clin Immunol. 1984; 74:524–535. PMID: 6208231.
15. Eifan AO, Akkoc T, Yildiz A, Keles S, Ozdemir C, Bahceciler NN, et al. Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/rhinitis children sensitized to house dust mite: an open randomized controlled trial. Clin Exp Allergy. 2010; 40:922–932. PMID: 20100188.

16. Röder E, Berger MY, de Groot H, Gerth van Wijk R. Sublingual immunotherapy in youngsters: adherence in a randomized clinical trial. Clin Exp Allergy. 2008; 38:1659–1667. PMID: 18631346.

17. Passalacqua G, Musarra A, Pecora S, Amoroso S, Antonicelli L, Cadario G, et al. Quantitative assessment of the compliance with a once-daily sublingual immunotherapy regimen in real life (EASY Project: Evaluation of A novel SLIT formulation during a Year). J Allergy Clin Immunol. 2006; 117:946–948. PMID: 16630956.

18. Cox L. Sublingual immunotherapy - Is there a role? Is the US ready for sublingual immunotherapy? [Internet]. Miami (FL): World Allergy Organization;2006. cited 2006 Mar 3. Available from: http://www.worldallergy.org/educational_programs/world_allergy_forum/miamibeach2006/cox.php.
19. Mun SJ, Shin JM, Han DH, Kim JW, Kim DY, Lee CH, et al. Efficacy and safety of a once-daily sublingual immunotherapy without escalation regimen in house dust mite-induced allergic rhinitis. Int Forum Allergy Rhinol. 2013; 3:177–183. PMID: 23044892.

20. Smith H, White P, Annila I, Poole J, Andre C, Frew A. Randomized controlled trial of high-dose sublingual immunotherapy to treat seasonal allergic rhinitis. J Allergy Clin Immunol. 2004; 114:831–837. PMID: 15480323.

21. Di Lorenzo G, Mansueto P, Pacor ML, Rizzo M, Castello F, Martinelli N, et al. Evaluation of serum s-IgE/total IgE ratio in predicting clinical response to allergen-specific immunotherapy. J Allergy Clin Immunol. 2009; 123:1103–1110. 1110.e1–1110.e4. PMID: 19356792.

22. Caffarelli C, Cardinale F, Povesi C, Chinellato I, Sterpeto Loffredo M. Optimal duration of SLIT. Int J Immunopathol Pharmacol. 2009; 22:17–22. PMID: 19944005.
Table 1
Demographics of patients with house dust mite-induced allergic rhinitis who were treated with SLIT (N=164)
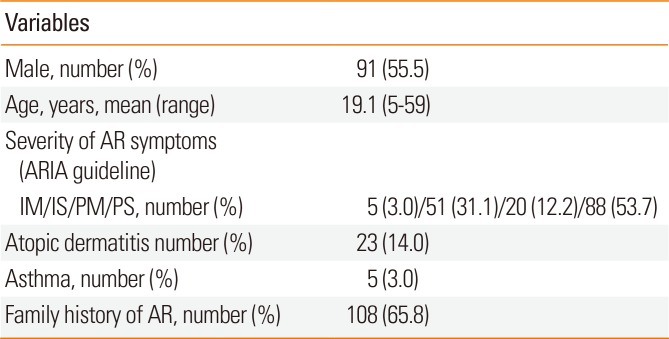
Atopic dermatitis or asthma, symptomatic asthma or atopic dermatitis that does not require regular medication such as an oral or inhaled steroid or anti-histamine.
AR, allergic rhinitis; ARIA, Allergic Rhinitis and Its Impact on Asthma; IM, intermittent mild; IS, intermittent moderate to severe; PM, persistent mild; PS, persistent moderate to severe.
Table 2
Scoring of systemic reactions to immunotherapy

Table 3
Effective response ratio
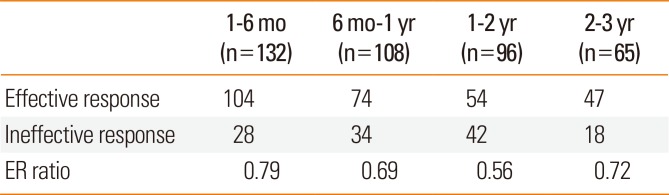
| 1-6 mo (n=132) | 6 mo-1 yr (n=108) | 1-2 yr (n=96) | 2-3 yr (n=65) | |
|---|---|---|---|---|
| Effective response | 104 | 74 | 54 | 47 |
| Ineffective response | 28 | 34 | 42 | 18 |
| ER ratio | 0.79 | 0.69 | 0.56 | 0.72 |
Table 4
Adverse events in the induction phase based on the European Academy of Allergology and Clinical Immunology criteria (N=164)
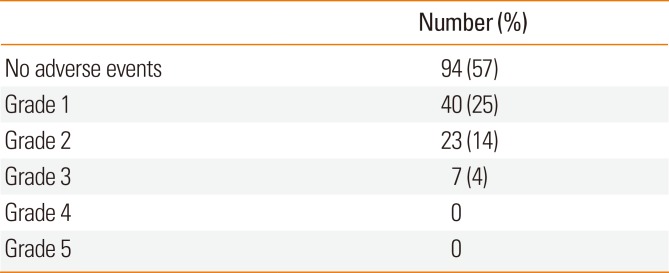
| Number (%) | |
|---|---|
| No adverse events | 94 (57) |
| Grade 1 | 40 (25) |
| Grade 2 | 23 (14) |
| Grade 3 | 7 (4) |
| Grade 4 | 0 |
| Grade 5 | 0 |
Table 5
Adverse events throughout the entire period (N=164)
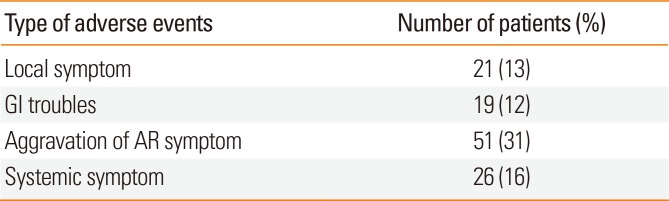
| Type of adverse events | Number of patients (%) |
|---|---|
| Local symptom | 21 (13) |
| GI troubles | 19 (12) |
| Aggravation of AR symptom | 51 (31) |
| Systemic symptom | 26 (16) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download