Abstract
Nitric oxide (NO) is a biologic mediator of various physiologic functions. Recent evidence suggests the clinical utility of fractional exhaled NO (FeNO) as a biomarker for assessing asthma and other respiratory diseases. FeNO methodologies have been recently standardized by international research groups and subsequently validated in several Korean population studies. Normal ranges for FeNO have been reported for various ethnic groups, and the clinical utility has been widely evaluated in asthma and various respiratory diseases. Based on current evidence including most of Korean population data, this position paper aims to introduce the methodological considerations, and provide the guidance for the proper clinical application of FeNO measurements in Korean populations.
Airway inflammation is a key component in asthma and various respiratory diseases.1 Therefore, the assessment of inflammation can provide important clinical information for patient diagnosis and monitoring, or for predicting treatment responses. Bronchoscopy has been the traditional gold standard method for directly obtaining airway tissues or cells, but its invasiveness has limited its applicability.2 The induced sputum test is an alternative tool that was standardized during the 1990s as a safer method of assessing airway inflammation.3,4 However, induced sputum analysis is technically challenging, time consuming, and not always feasible.
In this context, exhaled NO measurement has gained substantial clinical and scientific interest. NO is a biologic mediator generated when L-arginine is oxidized by NO synthase (NOS).5,6 It is involved in various functions of the respiratory system, including physiologic roles as a vasodilator, bronchodilator, neurotransmitter, and immune regulator.7 Although the specific contributions of NO to the pathophysiology of respiratory diseases remains unclear, patients with asthma exhale high concentrations of NO and show high airway epithelial expression of inducible NOS.8 Moreover, exhaled NO levels are reduced by corticosteroid therapy in asthma patients.9 Therefore, exhaled NO (FeNO) has been proposed as a useful biomarker reflecting respiratory eosinophilic inflammation, particularly in asthma.10,11
Over the last decade, an expanding literature on the clinical application of exhaled NO measurements has widened the clinical application of this non-invasive, simple, and reproducible measure. The American Thoracic Society (ATS) and the European Respiratory Society (ERS) have jointly published guidelines on the standardization and interpretation of exhaled NO measurement.10,12 Here, we reviewed current key literature and summarized relevant clinical studies conducted in Korean populations. This position paper aimed to introduce the standardized methods for NO assessment and to suggest its proper clinical applications.
FeNO indications10,13 can be classified into diagnostic and monitoring entities. FeNO is an attractive test as it is completely noninvasive, and provides immediate results. FeNO is a well-established tool in asthma research, with over 2,000 publications on its application. Its measurement adds a new dimension to the traditional clinical tools used in the assessment and management of airways diseases.
FeNO measurements in asthma have been used for the indications listed in Table 1. The current major indications include (1) detecting eosinophilic airway inflammation, (2) determining the likelihood of corticosteroid responsiveness, (3) monitoring airway inflammation to determine the potential need for corticosteroid therapy, and (4) unmasking otherwise unsuspected non-adherence to corticosteroid therapy.10,13 FeNO is also an effective diagnostic and monitoring tool for chronic cough,14 acute eosinophilic asthma,15 sinusitis, nasal polyposis, primary ciliary dyskinesia, chronic obstructive pulmonary disease (COPD), interstitial lung disease, cystic fibrosis, and other pulmonary or extrapulmonary diseases. However, additional research is required to validate its clinical utility. Impediments to the clinical application of FeNO include the paucity of publications on the utility of this marker in predicting exacerbations, improving long-term asthma control,16,17,18,19 or reducing health care costs.
At present, chemiluminescent and electrochemical detection methods have been mainly utilized in Korean population studies. Chemiluminescence is an established, standard technique and was recommended by ATS/ERS in 2005.12 Chemiluminescence devices measure NO levels by means of light generation from a chemical reaction of FeNO with ozone:20 NO + O3 → NO2 + O2 + photons. Light emitted by NO2 at >600 nm wavelength, which is highly proportional to NO concentration, is then detected by a photomultiplier tube.20 This technique has high sensitivity, with detection limits at the ppb-level (1.5 ppb for NIOX, Aerocrine, Solna, Sweden; and 0.5 ppb for Sievers NOA 280i, GE Analytical Instruments).20 However, the devices also have drawbacks such as heavy weight (20-50 kg), a large size and being less user-friendly than other devices. Moreover, there are costs associated with annual maintenance.20 The ANALYZER CLD 88 sp® (ECO MEDICS, Duernten, Switzerland) is another chemiluminescence device which recently became available for use with infants, children, and adults. This latter analyzer was utilized in a Korean study evaluating healthy newborns.21
Electrochemical sensor devices (NIOX MINO®,22 Aerocrine, Solna, Sweden; NObreath,23 Bedfont Scientific Ltd, UK) are recently introduced alternatives to chemiluminescence analyzers. These devices have a detection limit of 5 ppb but also the distinct advantages of being small, light-weight (less than 1 kg), easy-to-use, and hand-held. Another strong point is that electrochemical sensors do not require pre-test calibration; rather, they use an electrochemical sensor which needs to be replaced every 100-300 tests.20 In this regard, the electrochemical analyzers have promising roles in clinical practice. However, despite lower investment costs than chemiluminescence methods, the electrochemical devices have additional costs for each test and for periodic sensor replacement.
There appears to be a high degree of correlation between these 2 FeNO detection methods.24,25 However, electrochemical sensor devices generally report 4-10 ppb lower than chemiluminescent detectors.20,28 Such small differences are generally accepted as being not clinically relevant, but the analyzer device model should be clearly described when reporting the results.
All subjects must refrain from strenuous physical exercise and avoid eating, drinking or smoking for at least 1 hour before the assessment.26 Caution should be taken, since exhaled NO levels may increase after eating foods containing nitrates.27 Conversely, smoking causes acute and chronic reductions in NO levels,28,29 thus short- and long-term, active and passive smoking history should be obtained.12
Respiratory tract infections may increase exhaled NO levels in asthma.30 Therefore, NO assessment tests should be postponed until after recovery if possible. Exhaled NO levels may be decreased by inhaled or systemic corticosteroids,31 or by leukotriene antagonists.32 In addition, all patient medication details should be obtained to identify anything that may influence the results. Since circadian rhythms may affect NO levels,33 serial NO measurements should be performed at approximately the same time of the day.12
The most important factor to consider during the procedure is to maintain a 'constant flow rate'. In a flow-concentration curve, NO levels vary widely according to the expiratory rate.20 Accordingly, the ERS/ATS set the standard expiratory flow rate for NO measurement at 50 mL/s.12 A single-breath method is also used in all cooperative children. However, in uncooperative children, assessment by well-trained and experienced staff with adequate practice time, audiovisual aids, and dynamic flow restrictors is recommended. Alternatively, exhaled NO can be measured during spontaneous tidal breathing, which can be used in preschool children or infants.37
Subjects are instructed to sit in an upright position, exhale to residual volume, insert a mouth piece, inhale to total lung capacity, and then exhale immediately for 10 seconds at a constant flow rate of 50 mL/s. The investigators select the plateau period, and the plateau concentration is displayed by the instruments. FeNO measurements are repeated until 2 values are obtained that vary by less than 10%. The mean value is then recorded.
Subjects are instructed to sit in the upright position without a nose clip, exhale to residual volume, insert a mouth piece, inhale to total lung capacity through a nitric oxide-free filter connected to the instrument, and then exhale immediately for 10 seconds at a constant pressure guided by a visual cue in order to stabilize expiratory flow rate at 50 mL/s. FeNO levels are assessed along a stable plateau of exhaled NO concentration at least 3 seconds in length.
In general, NO measurement protocols should follow the current standard guidelines published by the ERS/ATS in 2005.12 For reporting, the minimum parameters to include are data, time, age, sex, race, height, weight, smoking status, prior diagnosis (particularly airway diseases), reason for testing, current corticosteroid medication (inhaled or systemic), and analyzer model used in the assessments.
We recommend following the current standardized procedures and validated instruments as stated by the ATS/ERS official documents.12
We also recommend that practitioners understand the characteristics of each analyzer and be familiar with the factors that can potentially influence FeNO levels.
Since FeNO levels distribution curves are positively skewed, it is reasonable to use logarithmic transformation for statistical analysis. Geometric means rather than arithmetic means are preferred to describe the skewed values. However, many investigators have used the arithmetic mean and standard deviation to describe FeNO values, and the ATS guidelines do not provide additional recommendations or guidance on this statistical issue. Thus, it should be noted that some of our present data have been provided as being transformed from their original statistical analyses.
Recent studies have reported normal ranges for adult populations in Korea (Table 2). However, the reported NO level distributions are skewed and show considerable overlap between control subjects and stable asthmatics.10 Moreover, the suggested 'normal' ranges vary considerably between study populations and analytic instruments used in the study.38,39,40,41,42,43 In this regard, ATS guidelines recommend the use of cutoff points rather than reference values based on the specific clinical setting and research question being addressed.10
The difficulty in determining normal ranges for FeNO may be attributed to several confounding factors. In the literature, race, age, sex, weight, height, smoking, and atopy have all been suggested as potential confounding factors. Amongst them, race, sex, atopy, age, and smoking have been regarded as major factors influencing FeNO measurement in adults. Asians are reported to have higher FeNO levels than other ethnic groups, presumably due to dietary habits or genetic variations in NOS genes.27,44 Among Korean adults, male sex has been positively associated with higher NO levels (Figure).45,46 Other population studies also reported sex differences in NO levels.40,41,47,48 The effects of atopy also have been frequently reported41,43,46,49,50; however, a recent study conducted in an unselected Korean older adult population (mean age: 59.9 years) did not confirm previous findings on atopy.45 The discrepancy in the results on atopy between 2 Korean population studies may be attributed to the effects of age, or also possibly to the analytic devices. Regarding obesity, a small study reported possible associations between FeNO and body mass index (BMI) in healthy non-smoking adults,51 but these results were not replicated in Koreans.52 Therefore, the heterogeneity in study design should be considered when interpreting suggested ranges for exhaled NO levels. Overall, both gender and atopy appear to be potentially significant confounding factors impacting FeNO levels in Korean adults.
A few studies have reported normal ranges in healthy children.53,54,55,56,57 In a recent multicenter study that enrolled 405 healthy children in Europe and the US aged 4 to 16 years, the geometric mean FeNO was 9.7 ppb, and the upper 95% confidence interval (CI) was 25.2 ppb. FeNO levels significantly increased with age (15-25 ppb for the upper 95% CI, depending on age), but showed no significant gender difference (geometric mean, 10.0 ppb in boys vs 9.4 ppb in girls; P=0.27).54 The ATS guidelines strongly recommend accounting for age as a factor impacting FeNO levels in children younger than 12 years of age.10 Another study of 114 healthy, non-atopic children in Finland reported that FeNO levels were significantly associated with age, height, weight, and body surface area. Using a skin prick test, the mean FeNO level for children with atopy was higher than that for children without atopy, irrespective of the presence of respiratory symptoms (10.3 ppb in non-atopics vs 16.1 ppb in symptomatic atopics or 14.6 ppb asymptomatic atopics; P<0.0001 and P=0.005, respectively).55 Racial differences of mean FeNO levels in healthy children were reported for Canadian school-aged children. Asian-Canadians had a significantly higher mean FeNO level than whites (12.7 ppb vs 22.8 ppb, P<0.001).56 In a recent study that enrolled children in Taiwan aged 5 to 18 years, the geometric mean FeNO level and the upper 95% CI were 11.2 ppb and 30.2 ppb in children without allergic sensitization. Subjects with allergic sensitization had comparatively elevated FeNO (mean 17.7 ppb, upper 95% CI 74.8 ppb).57
In Korea, the geometric mean and upper 95% CI of FeNO in healthy, non-atopic subjects were 10.3 ppb and 10.7 ppb in elementary school children.58 Healthy atopic children also had higher mean FeNO levels (16.7 ppb) than those of non-atopic children. Individual FeNO values by age were described in Table 3. FeNO measurements in school children was reported as simple and reproducible,39,54 but its feasibility does depend on age, since measurements can be challenging in preschool children. Age may impact the assessment success rate (from 40% at 4 years and 60% at 5 years to 100% at school age).54
The ATS guidelines state that NO levels <25 ppb indicate a decreased likelihood of eosinophilic inflammation or corticosteroid responses, and that levels >50 ppb indicate the eosinophilic or atopic nature of airway inflammation but also the likelihood of a positive response to corticosteroid treatment.10 However, in patients with intermediate NO levels (25-50 ppb in adults), the interpretation of results should be cautious and made in clinical context.10
Among Korean adults, Jo et al. found the cutoff points of 30.5 ppb for males and 20.5 ppb for females to be useful for differentiating patients with asthma from controls (sensitivity 0.7 and specificity 0.9 in males, and sensitivity 0.8 and specificity 0.87 in females).45 No other published studies have evaluated the performance of FeNO cutoff points for identification of asthma in Korean adults. In non-asthmatic patients with chronic cough, Oh et al. reported that FeNO measurements have utility in excluding the diagnosis of eosinophilic bronchitis if FeNO levels are <31.7 ppb.14 For eosinophilic pneumonia, Lee et al. analyzed the diagnostic ability of FeNO for differentiating acute eosinophilic pneumonia from non-eosinophilic pneumonia. The authors found that the cutoff value of 23.5 ppb had high diagnostic utility for predicting acute eosinophilic pneumonia among patients with fever and pneumonic infiltrations (positive likelihood ratio, 5.05, and negative likelihood ratio, 0.16) (Table 4).
ATS guidelines recommend that for children, FeNO levels <20 ppb can be used to indicate a decreased likelihood of eosinophilic inflammation and corticosteroids response, and that >35 ppb may indicate likely eosinophilic inflammation in symptomatic patients but also responsiveness to corticosteroids.10 The guidelines also recommend that FeNO levels between 20 ppb and 35 ppb should be interpreted with caution and in line with their clinical context.
In Korean studies, Choi et al. reported that children with asthma had higher levels of FeNO than the control group (mean FeNO, 28.3 ppb vs 20 ppb, respectively) and that FeNO levels were negatively correlated with PC20 in a methacholine provocation test.59 Recently, Oh et al. reported that FeNO levels were higher in preschool children with persistent or late-onset wheezing than in children with transient wheezing or non-wheezers. The authors concluded that FeNO measurement may be a better marker for identifying preschool wheezing phenotypes than measurement of airway hyperresponsiveness or spirometry.17 Results from other studies are described in Table 4.
The intra-individual variation in FeNO levels is reported to be 10% (or up to 4 ppb) in healthy subjects39,60 vs 20% in asthmatics.39,60,61 Thus, ATS guidelines recommend that in patients with asthma and baseline levels of more than 50 ppb, a change in FeNO of >20% from baseline levels is indicative of a significant change over time or following treatment.10
We recommend the use of specific FeNO level cutoff points for specific disease conditions or in distinct populations rather than adopting universal reference ranges. The basis for this recommendation includes the effects of various confounders, the methodological heterogeneity in previous studies, and the lack of any large-scale studies presenting nationwide data.
We suggest that FeNO levels have utility in supporting a diagnosis of asthma or eosinophilic inflammation in both adults and children. Despite potential inter-ethnic differences, we determined that the cutoff points suggested by the ATS guidelines are applicable to both Korean adults and children at present. To summarize, FeNO levels >50 ppb in adults or >35 ppb in children indicate a high likelihood of asthma or eosinophilic inflammation. Conversely, FeNO levels <25 ppb in adults (or <20 ppb in children) indicate a decreased likelihood of asthma or eosinophilic inflammation. Intermediate levels (25-50 ppb in adults and 20-35 ppb in children) should be interpreted with caution and consideration for the specific clinical context.
We suggest that a change in FeNO of >20% indicates a significant, clinically relevant change in asthmatics with FeNO levels >50 ppb (>35 ppb in children) at baseline. Therefore, serial measurements can be used to both to monitor clinical disease course and to assess treatment responses in patients with asthma.
FeNO measurement is a straightforward procedure that is easy to perform and well tolerated by subjects. No safety issues have been identified with FeNO assessment methods.62
Airway inflammation is an important feature in the development and progression of asthma.63 FeNO levels are increased in asthmatic patients compared to normal controls as a result of NOS induction by pro-inflammatory cytokines.64,65 FeNO levels have also been positively correlated with eosinophil concentrations in serum, bronchoalveolar lavage fluid, bronchial biopsies, and induced sputum.18,19,66,67,68
The clinical utility of FeNO is now widely accepted.11 The diagnostic accuracy for asthma may be superior, or at least similar when compared to conventional diagnostic tools such as spirometry, induced sputum, or peak flow meters.69 It may also be utilized for predicting exacerbations,70 or the treatment responses to corticosteroids71 and leukotriene antagonists.32 Unlike induced sputum eosinophils, the role of FeNO to tailor the dose of inhaled corticosteroids has not been generally proven at present;72 however, a recent trial demonstrated the strategy as useful in reducing asthma exacerbations in pregnant asthmatics.73
In children, recent Korean studies suggested additional utility of FeNO tests in classifying subtypes of preschool wheezing and predicting the likelihood of wheezing at 8 years. Children with late-onset or persistent preschool wheezing had higher FeNO levels than transient wheezers or non-wheezers.17 FeNO values in preschool children were also reported to improve the ability of specific IgE measurement to predict asthma symptoms at 8 years.74
However, there exist pitfalls in the FeNO interpretations. A proportion of asthmatics do not show elevations in FeNO levels. The discrepancy may be related to several factors.11 Most of all, the heterogeneity in asthma phenotypes seems to be a major relevant factor, particularly in adults.75,76 A large proportion of asthmatics may be non-eosinophilic.77 Despite reports of increased FeNO concentrations in school children with atopic asthma, no significant differences in FeNO were identified in subjects without atopy, irrespective of asthma. Low FeNO values in children may help exclude atopic asthma but high levels may be caused by allergic sensitization in addition to asthma.36,68,78
Obesity may be another factor involved in the discrepancy. Consistent data indicate that asthma is closely associated with obesity,79,80,81,82,83 and obese patients with asthma usually have more severe symptoms than non-obese patients with asthma.68,84 Now obese asthma is regarded as a unique phenotype characterized by severe symptoms for a given degree of lung function impairment, poor asthma control or quality of life, poor treatment responses to corticosteroids, or lack of eosinophilic inflammation.68,84,85 Therefore, in an obesity-related subtype of asthma, the diagnostic accuracy of FeNO has not been clearly demonstrated.86
Preclinical in vitro and animal data have demonstrated NO involvement in bronchiolitis, but only a few studies have evaluated FeNO levels in children with RSV bronchiolitis.87,88 In one study, FeNO levels were significantly lower in infants with bronchiolitis than in healthy control infants or in preschool children with recurrent wheezing.89 However, the same study demonstrated that 3 months after an initial diagnosis of RSV bronchiolitis, FeNO was significantly higher in the affected children than in normal subjects.89 Future studies are required to investigate potential relationships between viral infections and subsequent asthma.
FeNO levels are abnormally low in patients with cystic fibrosis (CF) compared to patients without cystic fibrosis, patients with non-primary ciliary dyskinesia (PCD), or normal controls, but there are no significant differences between CF and PCD.68,90,91 The low levels of NO in CF are directly related to the absence of NOS2 expression in the airway epithelium, which supports the concept that NOS2 contributes much of the NO that is detectable in exhaled breath.90,91
Data on NO levels in children with CF-related bronchiectasis appear conflicting, with one study showing lower NO in CF than in controls, while other authors reported significantly higher NO with CF, but no significant difference in NO compared to healthy children.66,68,90,91 On the other hand, in children with PCD, very low levels of nasal NO are an as yet only partially explained phenotype, whereas the range of FeNO levels in these subjects show considerable overlap with healthy subjects.90,91 Until there is consensus on changes in NO in CF, nasal NO levels have not been recommended for use in routine clinical practice.
In systemic sclerosis, FeNO concentrations are lower in subjects with interstitial lung involvement than in those without lung involvement, whereas patients without pulmonary disease have higher FeNO levels than healthy subjects.92 However, due to the rareness of diffuse lung disease in children, there are currently no reported studies that evaluated FeNO levels in this population.93
In adults, a recent single center study has demonstrated the clinical utility of high FeNO levels (>23.5 ppb) in differentiating acute eosinophilic pneumonia from its non-eosinophilic form.15
Based on some reports, response to corticosteroids is likely to be greater in patients with COPD who have sputum eosinophilia or elevated FeNO.94 Significant correlations have also been reported between baseline FeNO and FEV1 improvement after 2 months following treatment with inhaled budesonide 800 mg/day.95 This finding raises the possibility that FeNO measurements might also be used to predict steroid responsiveness in COPD.94,95
In adults with chronic cough, FeNO measurements may be useful in identifying non-asthmatic eosinophilic bronchitis,14 but have more controversial utility in predicting inhaled corticosteroid responses.96,97 In a study evaluating Korean children, FeNO levels were not associated with the severity of eosinophilic bronchitis.98
The clinical utility of FeNO measurements is under investigation for various diseases.11 Its usefulness in asthma is now well accepted, despite several limitations. On the basis of current findings, we encourage the use of FeNO measurements in prospective clinical trials to further validate its utility as an assessment endpoint in various respiratory conditions.
Measurement of FeNO is an easy, safe, reproducible, and non-invasive method to evaluate airway inflammation in both adults and children. In recent years, FeNO has been widely used to diagnose and manage asthma. FeNO measurement is useful for screening, precise diagnosis, predicting and evaluating treatment response, and predicting exacerbation in asthmatic patients. Age, sex, height, race, smoking, food intake, and atopy may each affect FeNO levels as confounders. FeNO levels increase with age, especially in children younger than 12 years old; however, a relatively low measurement success rate was shown in preschool children.
Evidence-based cutoff points rather than reference ranges are more likely to have diagnostic significance. In asthmatic patients, personal best value and changes from baseline may be more clinically relevant and useful indicators for evaluating patient treatment requirements than the actual FeNO levels themselves. Thus, Individual assessment according to clinical conditions is essential. FeNO measurements in preschool children can help to classify specific wheezing phenotypes and to predict future asthma symptoms at later life. Moving forward, FeNO, as with spirometry, will most likely be used to evaluate airway inflammatory changes during inhalation or an exercise provocation test.
This work will help to improve the routine clinical utilization of FeNO measurements in the field. Large population-based studies need to be conducted, since they will provide essential information on normal FeNO values. For tailored patient management based on the phenotypes of asthma or recurrent wheezing, more detailed study designs and patient classifications are needed.
Figures and Tables
Figure
Schematic presentation of the distribution of fractional exhaled nitric oxide levels in an adult Korean population: (A) males and (B) females. The data were obtained from the studies conducted by Jo et al.45
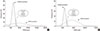
Table 1
Indications for FeNO measurement in asthma

Table 2
Normal ranges of exhaled nitric oxide among healthy Korean adults and children

| Reference | Study population | Groups | Ranges | Instruments |
|---|---|---|---|---|
| Adult studies | ||||
| Kim et al.46 | Nonsmoking healthy adults aged 20-68 yr from among employees at medical institutions (n=166, mean age 33 yr, atopy 48.2%) | Atopic male | Mean 37.3 ppb±SD 12.1 | Chemiluminescence analyzer (Sievers NOA280i, GE Analytical Instruments, Boulder, CO, USA) |
| Nonatopic male | Mean 33.9 ppb±SD 14.3 | |||
| Atopic female | Mean 28.6 ppb±SD 17.7 | |||
| Nonatopic female | Mean 24.1 ppb±SD 10.6 | |||
| Jo et al.45 | Healthy adults from the Ansung cohort (n=570, mean age 60 yr, atopy 23.3%) | Male | Geometric mean (95% CI) | Electrochemical sensor analyzer (NIOX MINO, Aerocrine, Sweden) |
| 15.5 ppb (14.4-16.8) | ||||
| Female | Geometric mean (95% CI) | |||
| 10.1 ppb (9.5-10.8) | ||||
| Children studies | ||||
| Cho et al.58 | Healthy children from 1 elementary school in Seoul (n=808, mean age 9.26 yr, atopy 38.0%) | Non-atopic | Geometric mean (95% CI) | Electrochemical sensor analyzer (NIOX MINO, Aerocrine, Sweden) |
| 10.3 ppb (9.9-10.7) | ||||
| Atopic | Geometric mean (95% CI) | |||
| 16.6 ppb (15.4-17.8) | ||||
| Kim et al.21 | Healthy newborns (n=41) from the nursery of 1 university hospital | Full term (n=31), Preterm (n=10) | Mean 10.0 ppb±SD 4.9, upper limit of 95% CI 19.8 | Chemiluminescence analyzer (CLD 88 exhalyzer) with tidal breathing technique |
Table 3
Individual FeNO values by age in elementary school children

Table 4
Summary of fractional exhaled nitric oxide levels in Koreans with various lung diseases

| Reference | Study population | Instrument | Findings | Conclusions |
|---|---|---|---|---|
| Adult studies | ||||
| Oh et al.14 | Chronic cough (>3 weeks), non-smokers (n=211) | Chemiluminescence analyzer (Sievers NOA280i) | 31.7 ppb to predict non-asthmatic eosinophilic bronchitis (sensitivity 86% and specificity 76%) | FeNO is useful for excluding non-asthmatic eosinophilic bronchitis |
| Jo et al.45 | Asthma (n=74) vs population-based controls (n=570) | Electrochemical sensor analyzer (NIOX MINO) | 30.5 ppb to predict asthma in males (sensitivity 70% and specificity 90%); 20.5 ppb for asthma in females (sensitivity 79.5% and specificity 86.9%) | FeNO is useful for differentiating asthmatics from controls |
| Park et al.19 | Asthma (n=90) vs non-asthma (n=71) among patients with respiratory symptoms | Electrochemical sensor analyzer (NIOX MINO) | 25.5 ppb to predict asthma among adults with suspected symptoms of asthma (sensitivity 57.1% and specificity 75.7%) | FeNO may be useful for diagnosing asthma and for assessing the level of asthma control |
| 24.5 ppb to predict uncontrolled asthma among asthmatics (sensitivity 76.6% and specificity 80.8%) | ||||
| Han et al.18 | Controlled asthma (n=34), partly controlled asthma (n=19), and uncontrolled asthma (n=18) | Chemiluminescence analyzer (Sievers NOA280i) | No significant difference in FeNO levels according to the asthma control status, or asthma control test score | FeNO levels are not associated with measures of asthma control in patients treated with inhaled corticosteroids |
| Lee et al.15 | Acute eosinophilic pneumonia (n=31) vs non-eosinophilic pneumonia (n=29) | Electrochemical sensor analyzer (NIOX MINO) | 23.5 ppb to predict acute eosinophilic pneumonia (sensitivity 87% and specificity 83%) | FeNO is useful for differentiating acute eosinophilic pneumonia in patients with fever and pulmonary infiltrates |
| Kwak et al.99 | Ventilator-associated pneumonia (n=19) vs controls (n=24) | Chemiluminescence analyzer (Sievers NOA280i) | 43.5 ppb to predict ventilator-associated pneumonia among adult patients with mechanical ventilation | FeNO may be useful for diagnosing ventilator-associated pneumonia |
| Children studies | ||||
| Oh et al.17 | Transient wheezers (n=67), persistent wheezers (n=23), late onset wheezers (n=29), and non-wheezers (n=282) from general preschool population aged 4 to 6 yr | Electrochemical sensor analyzer (NIOX MINO) | Mean±SD | FeNO may be a better marker for asthma phenotypes in preschool children than airway hyperresponsiveness or spirometric parameters |
| 14.4±9.2 ppb in persistent wheezers, 14.4±9.2 ppb for late-onset wheezers, 11.5±7.0 ppb for transient wheezers, 10.1±5.6 ppb for non-wheezers | ||||
| Choi et al.59 | Atopic asthma (n=98), non-atopic asthma (n=20), AR (n=79), and controls (n=74), children aged 6 to 15 yr | Chemiluminescence analyzer (CLD 88 exhalyzer) | Mean±SD | Patients with atopic asthma had higher FeNO levels than non-atopic asthmatics or controls |
| 48.33±32.28 ppb in atopic asthma, 15.92±5.99 ppb in non-atopic asthma 43.59±29.84 in allergic rhinitis | ||||
| Choi et al.100 | Patients with asthma (n=121), and controls (n=81), children aged 5 to 10 yr | Chemiluminescence analyzer (CLD 88 exhalyzer) | Median (interquartile range) | Patients with asthma had higher FeNO values than controls |
| 28.3 ppb (15.0-55.7) for asthma, 20.0 (12.3-39.7) for controls | ||||
| Seo et al.101 | Asthma (n=55), children aged 7 to 11 yr | Electrochemical sensor analyzer (NIOX MINO) | Geometric means (95% CI) | FeNO levels may decrease after methacholine-induced bronchoconstriction |
| 36.3 ppb (20.9-63.1) before MCT, 25.7 ppb (13.8-47.9) after MCT | Repeated spirometry maneuver was determined to have an effect on reducing FeNO levels | |||
| Baek et al.102 | Asthma with EIB (n=31), asthma without EIB (n=28), healthy controls (n=42) | Electrochemical sensor analyzer (NIOX MINO) | 1) Median (interquartile ranges) 26.0 ppb (15.0-46.0) in asthmatics with EIB, 16.0 ppb (12.5-28.0) in asthmatics without EIB, 12.0 ppb (10.0-15.3) in controls | Baseline FeNO levels correlate with the post-exercise decrease in FEV1 |
| 2) Cutoff value for EIB: 20 ppb (sensitivity 61.3%, specificity 80.0%) | FeNO measurement may be a tool for predicting EIB | |||
| Kim et al.16 | Atopic asthma (n=126), controls (n=30), children aged 8 to 16 yr | Electrochemical sensor analyzer (NIOX MINO) | Geometric means (95% CI) | FeNO was not related to spirometry values or scores for asthma control |
| 16.1 ppb (14.5-17.8) for atopic asthma, 7.5 ppb (7.0-8.1) for controls | ||||
| Woo et al.103 | Atopic asthma (n=129), non-atopic asthma (n=38), atopics without asthma (n=60), non-atopics without asthma (n=18), children aged 8 to 16 yr | Electrochemical sensor analyzer (NIOX MINO) | Geometric means (95% CI) | FeNO value in atopics was higher than that in non-atopics regardless of asthma. In atopic subjects, FeNO value was significantly higher in asthmatics than that in non-asthmatics. In contrast, in non-atopics, there was no difference in FeNO between asthmatics and non-asthmatics |
| 23.4 ppb (20.9-26.2) for asthmatics and 12.6 ppb (10.9-14.5) for non-asthmatics (P<0.001) | ||||
| 22 ppb to predict asthma among children with suspected symptoms of asthma (sensitivity 56.9% and specificity 87.2%) |
ACKNOWLEDGMENTS
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry for Health and Welfare, Republic of Korea (A092076).
References
1. Shelhamer JH, Levine SJ, Wu T, Jacoby DB, Kaliner MA, Rennard SI. NIH conference. Airway inflammation. Ann Intern Med. 1995; 123:288–304.
2. Vijverberg SJ, Koenderman L, Koster ES, van der Ent CK, Raaijmakers JA, Maitland-van der Zee AH. Biomarkers of therapy responsiveness in asthma: pitfalls and promises. Clin Exp Allergy. 2011; 41:615–629.
3. Pin I, Gibson PG, Kolendowicz R, Girgis-Gabardo A, Denburg JA, Hargreave FE, Dolovich J. Use of induced sputum cell counts to investigate airway inflammation in asthma. Thorax. 1992; 47:25–29.
4. Djukanović R, Sterk PJ, Fahy JV, Hargreave FE. Standardised methodology of sputum induction and processing. Eur Respir J Suppl. 2002; 37:1s–2s.
5. Spitale N, Popat N, McIvor A. Update on exhaled nitric oxide in pulmonary disease. Expert Rev Respir Med. 2012; 6:105–115.
6. Hamid Q, Springall DR, Riveros-Moreno V, Chanez P, Howarth P, Redington A, Bousquet J, Godard P, Holgate S, Polak JM. Induction of nitric oxide synthase in asthma. Lancet. 1993; 342:1510–1513.
7. Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994; 78:915–918.
8. Guo FH, Comhair SA, Zheng S, Dweik RA, Eissa NT, Thomassen MJ, Calhoun W, Erzurum SC. Molecular mechanisms of increased nitric oxide (NO) in asthma: evidence for transcriptional and post-translational regulation of NO synthesis. J Immunol. 2000; 164:5970–5980.
9. Silkoff PE, McClean P, Spino M, Erlich L, Slutsky AS, Zamel N. Dose-response relationship and reproducibility of the fall in exhaled nitric oxide after inhaled beclomethasone dipropionate therapy in asthma patients. Chest. 2001; 119:1322–1328.
10. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR. American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011; 184:602–615.
11. Kim SH, Yoon HJ. Use of the exhaled nitric oxide for management of asthma and respiratory diseases. Korean J Med. 2008; 74:579–586.
12. American Thoracic Society. European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 171:912–930.
13. Silkoff PE, Erzurum SC, Lundberg JO, George SC, Marczin N, Hunt JF, Effros R, Horvath I. American Thoracic Society. HOC Subcommittee of the Assembly on Allergy, Immunology, and Inflammation. ATS workshop proceedings: exhaled nitric oxide and nitric oxide oxidative metabolism in exhaled breath condensate. Proc Am Thorac Soc. 2006; 3:131–145.
14. Oh MJ, Lee JY, Lee BJ, Choi DC. Exhaled nitric oxide measurement is useful for the exclusion of nonasthmatic eosinophilic bronchitis in patients with chronic cough. Chest. 2008; 134:990–995.
15. Lee JE, Rhee CK, Lim JH, Lee SM, Shim YS, Lee CT, Lee SW. Fraction of exhaled nitric oxide in patients with acute eosinophilic pneumonia. Chest. 2012; 141:1267–1272.
16. Kim JO, Woo SI, Hahn YS. Relevance of exhaled nitric oxide levels to asthma control test scores and spirometry values in children with atopic asthma. Pediatr Allergy Respir Dis. 2011; 21:24–31.
17. Oh MA, Shim JY, Jung YH, Seo JH, Young Kim H, Kwon JW, Kim BJ, Kim HB, Kim WK, Lee SY, Jang GC, Song DJ, Kim HJ, Shin YJ, Park JW, Cho SH, Lee JS, Hong SJ. Fraction of exhaled nitric oxide and wheezing phenotypes in preschool children. Pediatr Pulmonol. 2013; 48:563–570.
18. Han CH, Park YI, Kwak HJ, Kim SI, Kim TH, Sohn JW, Yoon HJ, Shin DH, Park SS, Kim SH. Relationship between exhaled nitric oxide and levels of asthma control in asthma patients treated with inhaled corticosteroid. Tuberc Respir Dis. 2011; 71:106–113.
19. Park SH, Kim DH, Koh YI. Usefulness of fractional exhaled nitric oxide for the diagnosis of asthma and its assessment of asthma control. Korean J Asthma Allergy Clin Immunol. 2012; 32:83–91.
20. Cristescu SM, Mandon J, Harren FJ, Meriläinen P, Högman M. Methods of NO detection in exhaled breath. J Breath Res. 2013; 7:017104.
21. Kim SK, Rha YH, Bae CW. Measurements of exhaled nitric oxide in newborns. J Korean Soc Neonatol. 2007; 14:39–45.
22. Hemmingsson T, Linnarsson D, Gambert R. Novel hand-held device for exhaled nitric oxide-analysis in research and clinical applications. J Clin Monit Comput. 2004; 18:379–387.
23. Antus B, Horvath I, Barta I. Assessment of exhaled nitric oxide by a new hand-held device. Respir Med. 2010; 104:1377–1380.
24. Kim SH, Moon JY, Kwak HJ, Kim SI, Park DW, Kim JW, Kim TH, Sohn JW, Shin DH, Park SS, Yoon HJ. Comparison of two exhaled nitric oxide analyzers: the NIOX MINO hand-held electrochemical analyzer and the NOA280i stationary chemiluminescence analyzer. Respirology. 2012; 17:830–834.
25. Pisi R, Aiello M, Tzani P, Marangio E, Olivieri D, Chetta A. Measurement of fractional exhaled nitric oxide by a new portable device: comparison with the standard technique. J Asthma. 2010; 47:805–809.
26. Kippelen P, Caillaud C, Robert E, Masmoudi K, Préfaut C. Exhaled nitric oxide level during and after heavy exercise in athletes with exercise-induced hypoxaemia. Pflugers Arch. 2002; 444:397–404.
27. Olin AC, Aldenbratt A, Ekman A, Ljungkvist G, Jungersten L, Alving K, Torén K. Increased nitric oxide in exhaled air after intake of a nitrate-rich meal. Respir Med. 2001; 95:153–158.
28. Persson MG, Zetterström O, Agrenius V, Ihre E, Gustafsson LE. Single-breath nitric oxide measurements in asthmatic patients and smokers. Lancet. 1994; 343:146–147.
29. Kharitonov SA, Robbins RA, Yates D, Keatings V, Barnes PJ. Acute and chronic effects of cigarette smoking on exhaled nitric oxide. Am J Respir Crit Care Med. 1995; 152:609–612.
30. Kharitonov SA, Yates D, Barnes PJ. Increased nitric oxide in exhaled air of normal human subjects with upper respiratory tract infections. Eur Respir J. 1995; 8:295–297.
31. Kharitonov SA, Yates DH, Chung KF, Barnes PJ. Changes in the dose of inhaled steroid affect exhaled nitric oxide levels in asthmatic patients. Eur Respir J. 1996; 9:196–201.
32. Bisgaard H, Loland L, Øj JA. NO in exhaled air of asthmatic children is reduced by the leukotriene receptor antagonist montelukast. Am J Respir Crit Care Med. 1999; 160:1227–1231.
33. Mattes J, Storm van's Gravesande K, Moeller C, Moseler M, Brandis M, Kuehr J. Circadian variation of exhaled nitric oxide and urinary eosinophil protein X in asthmatic and healthy children. Pediatr Res. 2002; 51:190–194.
34. Deykin A, Halpern O, Massaro AF, Drazen JM, Israel E. Expired nitric oxide after bronchoprovocation and repeated spirometry in patients with asthma. Am J Respir Crit Care Med. 1998; 157:769–775.
35. Yates DH, Kharitonov SA, Barnes PJ. Effect of short- and long-acting inhaled beta2-agonists on exhaled nitric oxide in asthmatic patients. Eur Respir J. 1997; 10:1483–1488.
36. Kissoon N, Duckworth LJ, Blake KV, Murphy SP, Lima JJ. Effect of beta2-agonist treatment and spirometry on exhaled nitric oxide in healthy children and children with asthma. Pediatr Pulmonol. 2002; 34:203–208.
37. Baraldi E, de Jongste JC. European Respiratory Society/American Thoracic Society (ERS/ATS) Task Force. Measurement of exhaled nitric oxide in children, 2001. Eur Respir J. 2002; 20:223–237.
38. Dupont LJ, Rochette F, Demedts MG, Verleden GM. Exhaled nitric oxide correlates with airway hyperresponsiveness in steroid-naive patients with mild asthma. Am J Respir Crit Care Med. 1998; 157:894–898.
39. Kharitonov SA, Gonio F, Kelly C, Meah S, Barnes PJ. Reproducibility of exhaled nitric oxide measurements in healthy and asthmatic adults and children. Eur Respir J. 2003; 21:433–438.
40. Olivieri M, Talamini G, Corradi M, Perbellini L, Mutti A, Tantucci C, Malerba M. Reference values for exhaled nitric oxide (reveno) study. Respir Res. 2006; 7:94.
41. Travers J, Marsh S, Aldington S, Williams M, Shirtcliffe P, Pritchard A, Weatherall M, Beasley R. Reference ranges for exhaled nitric oxide derived from a random community survey of adults. Am J Respir Crit Care Med. 2007; 176:238–242.
42. Dressel H, de la Motte D, Reichert J, Ochmann U, Petru R, Angerer P, Holz O, Nowak D, Jörres RA. Exhaled nitric oxide: independent effects of atopy, smoking, respiratory tract infection, gender and height. Respir Med. 2008; 102:962–969.
43. Ko FW, Leung TF, Wong GW, Chu JH, Sy HY, Hui DS. Determinants of, and reference equation for, exhaled nitric oxide in the Chinese population. Eur Respir J. 2013; 42:767–775.
44. Wong GW, Liu EK, Leung TF, Yung E, Ko FW, Hui DS, Fok TF, Lai CK. High levels and gender difference of exhaled nitric oxide in Chinese schoolchildren. Clin Exp Allergy. 2005; 35:889–893.
45. Jo EJ, Song WJ, Kim TW, Park HW, Chang YS, Kim TB, Kim SH, Hur GY, Lee JH, Yoon HJ, Park HS, Cho NH, Moon HB, Min KU, Cho SH. Reference ranges and determinant factors for exhaled nitric oxide in a healthy Korean elderly population. Allergy Asthma Immunol Res. 2014; 6:504–510.
46. Kim SH, Kim TH, Sohn JW, Yoon HJ, Shin DH, Park SS. Reference values and determinants of exhaled nitric oxide in healthy Korean adults. J Asthma. 2010; 47:563–567.
47. Taylor DR, Mandhane P, Greene JM, Hancox RJ, Filsell S, McLachlan CR, Williamson AJ, Cowan JO, Smith AD, Sears MR. Factors affecting exhaled nitric oxide measurements: the effect of sex. Respir Res. 2007; 8:82.
48. Tsang KW, Ip SK, Leung R, Tipoe GL, Chan SL, Shum IH, Ip MS, Yan C, Fung PC, Chan-Yeung M, Lam W. Exhaled nitric oxide: the effects of age, gender and body size. Lung. 2001; 179:83–91.
49. Chng SY, Van Bever HP, Lian D, Lee SX, Xu XN, Wang XS, Goh DY. Relationship between exhaled nitric oxide and atopy in Asian young adults. Respirology. 2005; 10:40–45.
50. Grob NM, Dweik RA. Exhaled nitric oxide in asthma. From diagnosis, to monitoring, to screening: are we there yet? Chest. 2008; 133:837–839.
51. De Winter-de Groot KM, Van der Ent CK, Prins I, Tersmette JM, Uiterwaal CS. Exhaled nitric oxide: the missing link between asthma and obesity? J Allergy Clin Immunol. 2005; 115:419–420.
52. Kim SH, Kim TH, Lee JS, Koo TY, Lee CB, Yoon HJ, Shin DH, Park SS, Sohn JW. Adiposity, adipokines, and exhaled nitric oxide in healthy adults without asthma. J Asthma. 2011; 48:177–182.
53. Franklin PJ, Taplin R, Stick SM. A community study of exhaled nitric oxide in healthy children. Am J Respir Crit Care Med. 1999; 159:69–73.
54. Buchvald F, Baraldi E, Carraro S, Gaston B, De Jongste J, Pijnenburg MW, Silkoff PE, Bisgaard H. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol. 2005; 115:1130–1136.
55. Malmberg LP, Petäys T, Haahtela T, Laatikainen T, Jousilahti P, Vartiainen E, Mäkelä MJ. Exhaled nitric oxide in healthy nonatopic school-age children: determinants and height-adjusted reference values. Pediatr Pulmonol. 2006; 41:635–642.
56. Kovesi T, Kulka R, Dales R. Exhaled nitric oxide concentration is affected by age, height, and race in healthy 9- to 12-year-old children. Chest. 2008; 133:169–175.
57. Yao TC, Lee WI, Ou LS, Chen LC, Yeh KW, Huang JL. PATCH Study Group. Reference values of exhaled nitric oxide in healthy Asian children aged 5 to 18 years. Eur Respir J. 2012; 39:378–384.
58. Cho HJ, Jung YH, Yang SI, Lee E, Kim HY, Seo JH, Kwon JW, Kim BJ, Lee SY, Song DJ, Jang GC, Shim JY, Hong SJ. Reference values and determinants of fractional concentration of exhaled nitric oxide (FeNO) in healthy children. Allergy Asthma Immunol Res. 2014; 6:169–174.
59. Choi BS, Kim KW, Lee YJ, Baek J, Park HB, Kim YH, Sohn MH, Kim KE. Exhaled nitric oxide is associated with allergic inflammation in children. J Korean Med Sci. 2011; 26:1265–1269.
60. Ekroos H, Karjalainen J, Sarna S, Laitinen LA, Sovijarvi AR. Short-term variability of exhaled nitric oxide in young male patients with mild asthma and in healthy subjects. Respir Med. 2002; 96:895–900.
61. Pijnenburg MW, Floor SE, Hop WC, De Jongste JC. Daily ambulatory exhaled nitric oxide measurements in asthma. Pediatr Allergy Immunol. 2006; 17:189–193.
62. Szefler SJ, Wenzel S, Brown R, Erzurum SC, Fahy JV, Hamilton RG, Hunt JF, Kita H, Liu AH, Panettieri RA Jr, Schleimer RP, Minnicozzi M. Asthma outcomes: biomarkers. J Allergy Clin Immunol. 2012; 129:S9–S23.
63. Malerba M, Radaeli A, Ragnoli B, Airo' P, Corradi M, Ponticiello A, Zambruni A, Grassi V. Exhaled nitric oxide levels in systemic sclerosis with and without pulmonary involvement. Chest. 2007; 132:575–580.
64. Lundberg JO, Nordvall SL, Weitzberg E, Kollberg H, Alving K. Exhaled nitric oxide in paediatric asthma and cystic fibrosis. Arch Dis Child. 1996; 75:323–326.
65. Warke TJ, Fitch PS, Brown V, Taylor R, Lyons JD, Ennis M, Shields MD. Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax. 2002; 57:383–387.
66. Lex C, Ferreira F, Zacharasiewicz A, Nicholson AG, Haslam PL, Wilson NM, Hansel TT, Payne DN, Bush A. Airway eosinophilia in children with severe asthma: predictive values of noninvasive tests. Am J Respir Crit Care Med. 2006; 174:1286–1291.
67. Silvestri M, Sabatini F, Sale R, Defilippi AC, Fregonese L, Battistini E, Biraghi MG, Rossi GA. Correlations between exhaled nitric oxide levels, blood eosinophilia, and airway obstruction reversibility in childhood asthma are detectable only in atopic individuals. Pediatr Pulmonol. 2003; 35:358–363.
68. Corradi M, Zinelli C, Caffarelli C. Exhaled breath biomarkers in asthmatic children. Inflamm Allergy Drug Targets. 2007; 6:150–159.
69. Smith AD, Cowan JO, Filsell S, McLachlan C, Monti-Sheehan G, Jackson P, Taylor DR. Diagnosing asthma: comparisons between exhaled nitric oxide measurements and conventional tests. Am J Respir Crit Care Med. 2004; 169:473–478.
70. Gelb AF, Flynn Taylor C, Shinar CM, Gutierrez C, Zamel N. Role of spirometry and exhaled nitric oxide to predict exacerbations in treated asthmatics. Chest. 2006; 129:1492–1499.
71. Little SA, Chalmers GW, MacLeod KJ, McSharry C, Thomson NC. Non-invasive markers of airway inflammation as predictors of oral steroid responsiveness in asthma. Thorax. 2000; 55:232–234.
72. Petsky HL, Cates CJ, Li A, Kynaston JA, Turner C, Chang AB. Tailored interventions based on exhaled nitric oxide versus clinical symptoms for asthma in children and adults. Cochrane Database Syst Rev. 2009; CD006340.
73. Powell H, Murphy VE, Taylor DR, Hensley MJ, McCaffery K, Giles W, Clifton VL, Gibson PG. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet. 2011; 378:983–990.
74. Caudri D, Wijga AH, Hoekstra MO, Kerkhof M, Koppelman GH, Brunekreef B, Smit HA, de Jongste JC. Prediction of asthma in symptomatic preschool children using exhaled nitric oxide, Rint and specific IgE. Thorax. 2010; 65:801–807.
75. de Nijs SB, Venekamp LN, Bel EH. Adult-onset asthma: is it really different? Eur Respir Rev. 2013; 22:44–52.
76. Kim TB, Jang AS, Kwon HS, Park JS, Chang YS, Cho SH, Choi BW, Park JW, Nam DH, Yoon HJ, Cho YJ, Moon HB, Cho YS, Park CS. COREA Study Group. Identification of asthma clusters in two independent Korean adult asthma cohorts. Eur Respir J. 2013; 41:1308–1314.
77. McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, Fahy JV. Asthma Clinical Research Network of the National Heart, Lung, and Blood Institute. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med. 2012; 185:612–619.
78. Yao TC, Ou LS, Lee WI, Yeh KW, Chen LC, Huang JL. PATCH study group. Exhaled nitric oxide discriminates children with and without allergic sensitization in a population-based study. Clin Exp Allergy. 2011; 41:556–564.
79. Song WJ, Kim SH, Lim S, Park YJ, Kim MH, Lee SM, Lee SB, Kim KW, Jang HC, Cho SH, Min KU, Chang YS. Association between obesity and asthma in the elderly population: potential roles of abdominal subcutaneous adiposity and sarcopenia. Ann Allergy Asthma Immunol. 2012; 109:243–248.
80. Kwon JW, Kim SH, Kim TB, Kim SH, Park HW, Chang YS, Jang AS, Cho YS, Nahm DH, Park JW, Yoon HJ, Cho YJ, Choi BW, Moon HB, Cho SH. Airway hyperresponsiveness is negatively associated with obesity or overweight status in patients with asthma. Int Arch Allergy Immunol. 2012; 159:187–193.
81. Kim KM, Kim SS, Kwon JW, Jung JW, Kim TW, Lee SH, Min KU, Kim YY, Cho SH. Association between subcutaneous abdominal fat and airway hyperresponsiveness. Allergy Asthma Proc. 2011; 32:68–73.
82. Yoo S, Kim HB, Lee SY, Kim BS, Kim JH, Yu JH, Kim BJ, Hong SJ. Association between obesity and the prevalence of allergic diseases, atopy, and bronchial hyperresponsiveness in Korean adolescents. Int Arch Allergy Immunol. 2011; 154:42–48.
83. Jang AS, Lee JH, Park SW, Shin MY, Kim DJ, Park CS. Severe airway hyperresponsiveness in school-aged boys with a high body mass index. Korean J Intern Med. 2006; 21:10–14.
84. Santamaria F, Montella S, Pietrobelli A. Obesity and pulmonary disease: unanswered questions. Obes Rev. 2012; 13:822–833.
85. Santamaria F, Montella S, Greco L, Valerio G, Franzese A, Maniscalco M, Fiorentino G, Peroni D, Pietrobelli A, De Stefano S, Sperli F, Boner AL. Obesity duration is associated to pulmonary function impairment in obese subjects. Obesity (Silver Spring). 2011; 19:1623–1628.
86. Berg CM, Thelle DS, Rosengren A, Lissner L, Torén K, Olin AC. Decreased fraction of exhaled nitric oxide in obese subjects with asthma symptoms: data from the population study INTERGENE/ADONIX. Chest. 2011; 139:1109–1116.
87. Kao YJ, Piedra PA, Larsen GL, Colasurdo GN. Induction and regulation of nitric oxide synthase in airway epithelial cells by respiratory syncytial virus. Am J Respir Crit Care Med. 2001; 163:532–539.
88. Song W, Liu G, Bosworth CA, Walker JR, Megaw GA, Lazrak A, Abraham E, Sullender WM, Matalon S. Respiratory syncytial virus inhibits lung epithelial Na+ channels by up-regulating inducible nitric-oxide synthase. J Biol Chem. 2009; 284:7294–7306.
89. Gadish T, Soferman R, Merimovitch T, Fireman E, Sivan Y. Exhaled nitric oxide in acute respiratory syncytial virus bronchiolitis. Arch Pediatr Adolesc Med. 2010; 164:727–731.
90. Ho LP, Innes JA, Greening AP. Exhaled nitric oxide is not elevated in the inflammatory airways diseases of cystic fibrosis and bronchiectasis. Eur Respir J. 1998; 12:1290–1294.
91. Robroeks CM, Rosias PP, van Vliet D, Jöbsis Q, Yntema JB, Brackel HJ, Damoiseaux JG, den Hartog GM, Wodzig WK, Dompeling E. Biomarkers in exhaled breath condensate indicate presence and severity of cystic fibrosis in children. Pediatr Allergy Immunol. 2008; 19:652–659.
92. Sandrini A, Johnson AR, Thomas PS, Yates DH. Fractional exhaled nitric oxide concentration is increased in asbestosis and pleural plaques. Respirology. 2006; 11:325–329.
93. Wilsher ML, Fergusson W, Milne D, Wells AU. Exhaled nitric oxide in sarcoidosis. Thorax. 2005; 60:967–970.
94. de Laurentiis G, Maniscalco M, Cianciulli F, Stanziola A, Marsico S, Lundberg JO, Weitzberg E, Sofia M. Exhaled nitric oxide monitoring in COPD using a portable analyzer. Pulm Pharmacol Ther. 2008; 21:689–693.
95. Dummer JF, Epton MJ, Cowan JO, Cook JM, Condliffe R, Landhuis CE, Smith AD, Taylor DR. Predicting corticosteroid response in chronic obstructive pulmonary disease using exhaled nitric oxide. Am J Respir Crit Care Med. 2009; 180:846–852.
96. Hahn PY, Morgenthaler TY, Lim KG. Use of exhaled nitric oxide in predicting response to inhaled corticosteroids for chronic cough. Mayo Clin Proc. 2007; 82:1350–1355.
97. Prieto L, Ferrer A, Ponce S, Palop J, Marín J. Exhaled nitric oxide measurement is not useful for predicting the response to inhaled corticosteroids in subjects with chronic cough. Chest. 2009; 136:816–822.
98. Kim YH, Kim KW, Baek J, Park HB, Kim H, Song KJ, Lee JM, Sohn MH, Kim KE. Usefulness of impulse oscillometry and fractional exhaled nitric oxide in children with Eosinophilic bronchitis. Pediatr Pulmonol. 2013; 48:221–228.
99. Kwak HJ, Kim SH, Kim TH, Yoon HJ, Shin DH, Park SS, Sohn JW. Exhaled nitric oxide in patients with ventilator associated pneumonia. Korean J Crit Care Med. 2012; 27:82–88.
100. Choi BS, Jee HM, Park YH, Kim KW, Sohn MH, Kim KE. Relationship between exhaled nitric oxide concentration and pulmonary function/airway hyperresponsiveness in asthmatic children. Pediatr Allergy Respir Dis. 2009; 19:291–299.
101. Seo HS, Chung BH, Park HN, Seo SC, Siegfried B, Song DJ, Choung JT, Yoo Y. Relationships between fraction of nitric oxide, airway hyperresponsiveness, blood eoshinophil counts and serum eosinophil cationic protein in asthmatic children. Pediatr Allergy Respir Dis. 2012; 22:282–291.
102. Baek HS, Park YR, Kim JH, Oh JW, Lee HB. Nitric oxide correlates with exercise-induced bronchoconstriction in asthmatic children. Pediatr Allergy Respir Dis. 2011; 21:99–107.
103. Woo SI, Lee JH, Kim H, Kang JW, Sun YH, Hahn YS. Utility of fractional exhaled nitric oxide (F(E)NO) measurements in diagnosing asthma. Respir Med. 2012; 106:1103–1109.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download