Abstract
This study examined the prevalence of oral microbes in the saliva of oncological patients and healthy subjects. PCR was used to assess the frequency of oral microbes including 3 cariogenic bacteria, 5 periodontopathic bacteria and 4 Candida species in the saliva of 104 oncological patients and 52 healthy subjects. Among these microorganims, Streptococcus mutans, Fusobacterium nucleatum and Candida albicans were most frequently detected in both groups. There were no significant differences in the prevalence of cariogenic bacteria between the patient and healthy groups, whereas significant differences in the frequency of Porphyromonas gingivalis and Tannerella forsythia were observed between the two groups (p < 0.05). The prevalence of all five periodontopathogens was higher in the healthy group than in the patient group. The prevalence of C. albicans in patients was significantly higher than that of healthy group (p < 0.05). In conclusion, there were significant differences in the prevalence of P. gingivalis, T. forsythia and C. albicans between the oncological patient group and healthy group.
REFERENCES
1). Krasse B. Human streptococci and experimental caries in hamsters. Arch Oral Biol. 1966. 11:429–36.

2). Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986. 50:353–80.
3). Alaluusua S., Renkonen OV. Streptococcus mutans establishment and dental caries experience in children from 2 to 4 years old. Scand J Dent Res. 1983. 91:453–7.
4). Kawamura Y., Hou XG., Sultana F., Miura H., Ezaki T. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int J Syst Bacteriol. 1995. 45:406–8.
5). Redondo-López V., Cook RL., Sobel JD. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis. 1990. 12:856–72.
6). Ahrne S., Nobaek S., Jeppsson B., Adlerberth I., Wold AE., Molin G. The normal Lactobacillus flora of healthy human rectal and oral mucosa. J Appl Microbiol. 1998. 85:88–94.
7). Haffajee AD., Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994. 5:78–111.

8). Slots J., Ting M. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: occurrence and treatment. Periodontol 2000. 1999. 20:82–121.
9). Haffajee AD., Teles RP., Socransky SS. Association of Eubacterium nodatum and Treponema denticola with human periodontitis lesions. Oral Microbiol Immunol. 2006. 21:269–82.
10). Akpan A., Morgan R. Oral candidiasis: a review. Postgrad Med J. 2002. 78:455–9.
11). Nagy K., Szöke I., Sonkodi I., Nagy E., Mari A., Szolnoky G., Newman HN. Inhibition of microflora associated with oral malignancy. Oral Oncol. 2000. 36:32–6.

12). de Soet JJ., van Dalen PJ., Pavicic MJ., de Graaff J. Enumeration of mutans streptococci in clinical samples by using monoclonal antibodies. J Clin Microbiol. 1990. 28:2467–72.

13). Beighton D., Russell RR., Whiley RA. A simple biochemical scheme for the differentiation of Streptococcus mutans and Streptococcus sobrinus. Caries Res. 1991. 25:174–8.
14). Cangelosi GA., Iversen JM., Zuo Y., Oswald TK., Lamont RJ. Oligonucleotide probes for mutans streptococci. Mol Cell Probes. 1994. 8:73–80.
15). Ashimoto A., Chen C., Bakker I., Slots J. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol Immunol. 1996. 11:266–73.

16). Sakamoto M., Takeuchi Y., Umeda M., Ishikawa I., Benno Y., Nakase T. Detection of Treponema socranskii associated with human periodontitis by PCR. Microbiol Immunol. 1999. 43:485–90.
17). Kang MS., Choi EK., Choi DH., Ryu SY., Lee HH., Kang HC., Koh JT., Kim OS., Hwang YC., Yoon SJ., Kim SM., Yang KH., Kang IC. Antibacterial activity of pyrrolidine dithiocarbamate. FEMS Microbiol Lett. 2008. 280:250–4.

18). Ohta K., Makinen KK., Loesche WJ. Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun. 1986. 53:213–20.
19). Choi MH., Yoo SY., Kang DW., Lim CK., Kook JK. Nested PCR for the detection of Streptococcus mutans. Korean J Microbiol. 2006. 42:19–25.
20). Yoo SY., Park SJ., Jeong DK., Kim KW., Lim SH., Lee SH., Choe SJ., Chang YH., Park I., Kook JK. Isolation and characterization of the mutans streptococci from the dental plaques in Koreans. J Microbiol. 2007. 45:246–55.
21). Byun R., Nadkarni MA., Chhour KL., Martin FE., Jacques NA., Hunter N. Quantitative analysis of diverse Lactobacillus species present in advanced dental caries. J Clin Microbiol. 2004. 42:3128–36.
22). Ahmad S., Khan Z., Mustafa AS., Khan ZU. Seminested PCR for diagnosis of candidemia: comparison with culture, antigen detection, and biochemical methods for species identification. J Clin Micrbiol. 2002. 40:2483–9.

23). Stephens LC., King GK., Peters LJ., Ang KK., Schultheiss TE., Jardine JH. Acute and late radiation injury in rhesus monkey parotid glands. Evidence of interphase cell death. Am J Pathol. 1986. 124:469–78.
24). Schubert MM., Izutsu KT. Iatrogenic causes of salivary gland dysfunction. J Dent Res. 1987. 66:680–8.

25). Tong HC., Gao XJ., Dong XZ. Non-mutans streptococci in patients receiving radiotherapy in the head and neck area. Caries Res. 2003. 37:261–6.

26). Brown LR., Dreizen S., Handler S., Johnston DA. Effect of radiation-induced xerostomia on human oral microflora. J Dent Res. 1975. 54:740–50.

27). Keene HJ., Fleming TJ., Toth BB. Cariogenic microflora in patients with Hodgkin's disease before and after mantle field radiotherapy. Oral Surg Oral Med Oral Pathol. 1994. 78:577–82.

29). Mandell RL. A longitudinal microbiological investigation of Actinobacillus actinomycetemcomitans and Eikenella corrodens in juvenile periodontitis. Infect Immun. 1984. 45:778–80.
30). Meyer DH., Fives-Taylor PM. The role of Actinobacillus actinomycetemcomitans in the pathogenesis of periodontal disease. Trends Microbiol. 1997. 5:224–8.
31). Kasuga Y., Ishihara K., Okuda K. Significance of detection of Porphyromonas gingivalis, Bacteroides forsythus and Treponema denticola in periodontal pockets. Bull Tokyo Dent Coll. 2000. 41:109–17.
32). Socransky SS., Haffajee AD., Cugini MA., Smith C., Kent RL Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998. 25:134–44.

33). van Winkelhoff AJ., Loos BG., van der Reijden WA., van der Velden U. Porphyromonas gingivalis, Bacteroides forsythus and other putative periodontal pathogens in subjects with and without periodontal destruction. J Clin Periodontol. 2002. 29:1023–8.
34). Müller HP., Heinecke A., Fuhrmann A., Eger T., Zöller L. Intraoral distribution of Actinobacillus actinomycetemcomitans in young adults with minimal periodontal disease. J Periodontal Res. 2001. 36:114–23.
35). Redding SW., Zellars RC., Kirkpatrick WR., McAtee RK., Caceres MA., Fothergill AW., Lopez-Ribot JL., Bailey CW., Rinaldi MG., Patterson TF. Epidemiology of oropharyngeal Candida colonization and infection in patients receiving radiation for head and neck cancer. J Clin Microbiol. 1999. 37:3896–900.
36). Wingard JR. Importance of Candida species other than C. albicans as pathogens in oncology patients. Clin Infect Dis. 1995. 20:115–25.
37). Safdar A., Chaturvedi V., Cross EW., Park S., Bernard EM., Armstrong D., Perlin DS. Prospective study of Candida species in patients at a comprehensive cancer center. Antimicrob Agents Chemother. 2001. 45:2129–33.
38). Sand L., Jalouli J., Larsson PA., Hirsch JM. Human papilloma viruses in oral lesions. Anticancer Res. 2000. 20:1183–8.
39). Dongari-Bagtzoglou A., Dwivedi P., Ioannidou E., Shaqman M., Hull D., Burleson J. Oral Candida infection and colonization in solid organ transplant recipients. Oral Microbiol Immunol. 2009. 24:249–54.
Figure 1.
The prevalence of cariogenic bacteria in the saliva of the study groups by PCR. HN, patients with head and neck tumors; HE, patients with hematological neoplasia; SO, patients with solid tumors; TO, total oncological patients; H, healthy subjects; Sm, S. mutans; Ss, S. sobrinus; Lb, Lactobacillus spp. ∗p < 0.05, vs. healthy subjects.
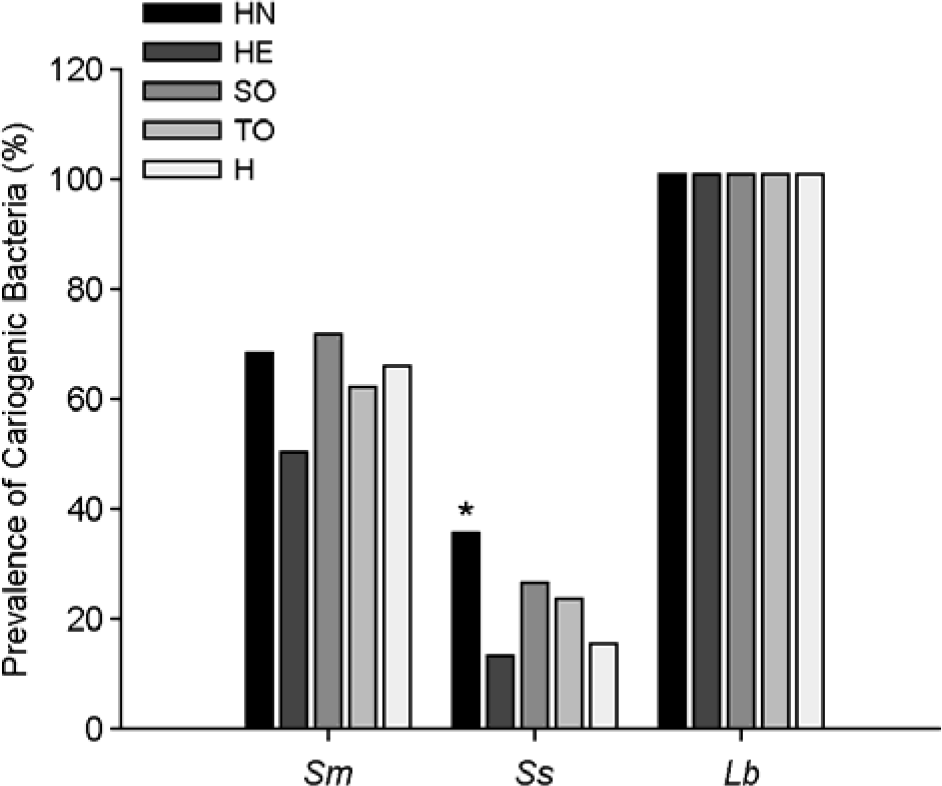
Figure 2.
The prevalence of periodontopathic bacteria in the saliva of the study groups by PCR. HN, patients with head and neck tumors; HE, patients with hematological neoplasia; SO, patients with solid tumors; TO, total oncological patients; H, healthy subjects; Aa, A. actinomycetemcomitans; Pg, P. gingivalis; Tf, T. forsythia; Fn, F. nucleatum; Td, T. denticola. ∗p < 0.05, vs. healthy subjects.
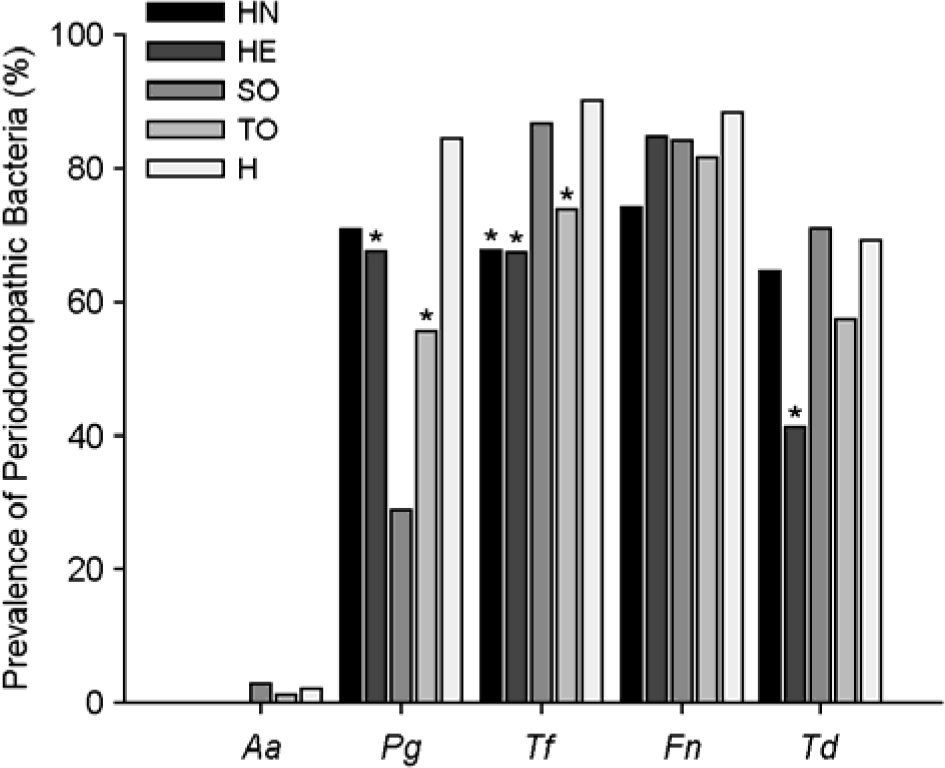
Figure 3.
The prevalence of Candida species in the saliva of the study groups by PCR. HN, patients with head and neck tumors; HE, patients with hematological neoplasia; SO, patients with solid tumors; TO, total oncological patients; H, healthy subjects; Ca, C. albicans; Cg, C. glabrata; Cp, C. parapsilosis; Ct, C. tropicalis. ∗p < 0.05, vs. healthy subjects.
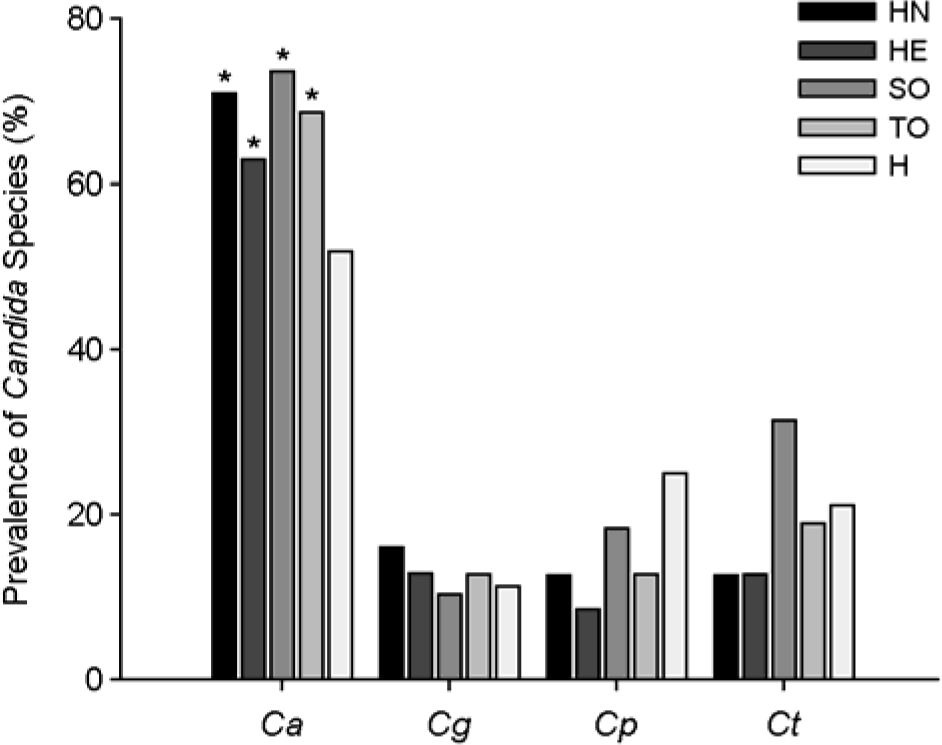
Table 1.
The species-specific primers used for PCR
| Oligonucleotide sequence (5′→3′) | Amplicon size (bp) | References |
|---|---|---|
| S. mutans | ||
| aF: TGGGACGCAAGAGAACACA | 356 | 19 |
| bR: GCGGCGTTGCTCGGTCAGA | ||
| S. sobrinus | ||
| F: CATTGGTAACACCGGACTTGC | 533 | 20 |
| R: CGCCTGCGCTCCCTTTAC | ||
| Lactobacillus spp. | ||
| F: TGGAAACAGATGCTAATACCG | 232 | 21 |
| R: GTCCATTGTGGAAGATTCCC | ||
| A. actinomycetemcomitans | ||
| F: AAACCCATCTCTGAGTTCTTCTTC | 557 | 15 |
| R: ATGCCAACTTGACGTTAAAT | ||
| F. nucleatum | ||
| F: GAAGAAACAAATGACGGTAACAAC | 705 | 15 |
| R: GTCATCCCCACCTTCCTCCT | ||
| P. gingivalis | ||
| F: AGGCAGCTTGCCATACTGCG | 404 | 15 |
| R: ACTGTTAGCAACTACCGATGT | ||
| T. forsythia | 641 | 15 |
| F: GCGTATGTAACCTGCCCGCA | ||
| R: TGCTTCAGTGTCAGTTATACCT | ||
| T. denticola | ||
| F: TAATACCGAATGTGCTCATTTACAT | 316 | 15 |
| R: TCAAAGAAGCATTCCCTCTTCTTCTTA | ||
| C. albicans | ||
| F: ATTGCTTGCGGCGGTAACGTCC | 110 | 22 |
| C. glabrata | ||
| F: TAGGTTTTACCAACTCGGTGTT | 160 | 22 |
| C. parapsilosis | ||
| F: ACAAACTCCAAAACTTCTTCCA | 100 | 22 |
| C. tropicalis | ||
| F: AACGCTTATTTTGCTAGTGGCC | 110 | 22 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download