Abstract
Staphylococcal cassette chromosome mec (SCCmec) type and coagulase serotype are important epidemiologic factors in methicillin-resistant Staphylococcus aureus (MRSA). To investigate correlation between SCCmec type and coagulase serotype of MRSA, we analyzed SCCmec types of MRSA strains isolated from clinical sources and compared the results to coagulase serotypes and antimicrobial susceptibility patterns. A total of 108 MRSA isolates were classified into four SCCmec types: II (55.6%), IV (21.3%) III (13.0%) and IIIA (8.3%), and five coagulase serotypes: II (54.6%), IV (21.3%), V (18.5%) and VII (2.8%). All of coagulase type II, IV and V strains belonged to SCCmec type II, III/IIIA and IV, respectively. SCCmec types II, III and IIIA were multidrug resistant, whereas SCCmec type IV strains were non-multidrug resistant except beta-lactams and erythromycin. The data provide that there is a significant correlation between SCCmec types and phenotypic characteristic of coagulase serotypes.
REFERENCES
1). Chamber HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001. 7:178–82.
2). Eady EA., Cove JH. Staphylococcal resistance revisited: community-acquired methicillin resistant Staphylococcus aureus-an emerging problem for the management of skin and soft tissue infections. Curr Opin Infect Dis. 2003. 16:103–24.
3). Lee YS., Kim HB., Yoo JI., Yang SJ., Sa CM., Choi YH., Kim BS. Molecular epidemiology of methicillin resistant Staphylococcus aureus in Korea. The Report of National Institute of Health. 1999. 36:67–76.
4). Kim JS., Kim HS., Song WK., Cho HC., Lee KM., Kim EC. Molecular epidemiology of methicillin-resistant Staphylococcus aureus isolates with toxic shock syndrome toxin and staphylococcal enterotoxin C genes. Korean J Lab Med. 2007. 27:118–23.
5). Daum RS., Ito T., Hiramatsu K., Hussain F., Mongkolrattanothai K., Jamklang M., Boyle-Vavra S. A novel methicillin-resistance cassette in community-acquired methicillin-resistant Staphylococcus aureus isolates of diverse genetic backgrounds. J Infect Dis. 2002. 186:1344–7.
6). Diep BA., Carleton HA., Chang RF., Sensabaugh GF., Perdreau-Remington F. Roles of 34 virulence genes in the evolution of hospital-and community-associated strains of methicillin-resistant Staphylococcus aureus. J Infect Dis. 2006. 193:1495–503.
7). Kilic A., Li H., Stratton CW., Tang YW. Antimicrobial susceptibility patterns and staphylococcal cassette chromosome mec types of as well as Panton-Valentine leukocidin occurrence among, methicillin-resistant Staphylococcus aureus isolates from children and adults in middle Tennessee. J Clin Microbiol. 2006. 44:4436–40.
8). Boyle-Vavra S., Ereshefsky B., Wang CC., Daum RS. Successful multiresistant community-associated methicillin-resistant Staphylococcus aureus lineage from Taipei, Taiwan, that carries either the novel Staphylococcal chromosome cassette mec (SCCmec) type VT or SCCmec type IV. J Clin Microbiol. 2005. 43:4719–30.
9). Ito T., Ma XX., Takeuchi F., Okuma K., Yuzawa H., Hiramatsu K. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob Agents Chemother. 2004. 48:2637–51.
10). Oliveira DC., de Lencastre H. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2002. 46:2155–61.
11). Hisata K., Kuwahara-Arai K., Yamanoto M., Ito T., Nakatomi Y., Cui L., Baba T., Terasawa M., Sotozono C., Kinoshita S., Yamashiro Y., Hiramatsu K. Dissemination of methicillin-resistant staphylococci among healthy Japanese children. J Clin Microbiol. 2005. 43:3364–72.

12). Liu Y., Wang H., Du N., Shen E., Chen H., Niu J., Ye H., Chen M. Molecular evidence for spread of two major methicillin-resistant Staphylococcus aureus clones with a unique geographic distribution in Chinese hospitals. Antimicrob Agents Chemother. 2009. 53:512–8.
13). Durand G., Bes M., Meugnier H., Enright MC., Forey F., Liassine N., Wenger A., Kikuchi K., Lina G., Vandenesch F., Etienne J. Detection of new methicillin-resistant Staphylococcus aureus clones containing the toxic shock syndrome toxin 1 gene responsible for hospital- and community-acquired infections in France. J Clin Microbiol. 2006. 44:847–53.
14). Johnson WM., Tyler SD., Ewan EP., Ashton FE., Pollard DR., Rozee KR. Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin 1 in Staphylococcus aureus by the polymerase chain reaction. J Clin Microbiol. 1991. 29:426–30.
15). Kanemitsu K., Yamamoto H., Takemura H., Kaku M., Shimada J. Relatedness between the coagulase gene 3′-end region and coagulase serotypes among Staphylococcus aureus strains. Microbiol Immunol. 2001. 45:23–7.
16). Watanabe S., Ito T., Takeuchi F., Endo M., Okuno E., Hiramatsu K. Structural comparison of ten serotypes of staphylocoagulases in Staphylococcus aureus. J Bacteriol. 2005. 187:3698–707.
17). Ishino K., Tsuchizaki N., Ishikawa J., Hotta K. Usefulness of PCR-restriction fragment length polymorphism typing of the coagulase gene to discriminate arbekacin-resistant methicillin-resistant Staphylococcus aureus strains. J Clin Microbiol. 2007. 45:607–9.
18). Murakami K., Minamide W., Wada K., Nakamura E., Teraoka H., Watanabe S. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol. 1991. 29:2240–4.

19). Hwang SM., Seki K., Sakurada J., Ogasawara M., Murai M., Ohmayu S., Kurosaka K., Masuda S. Improved methods for detection and serotyping of coagulase from Staphylococcus aureus. Microbiol Immunol. 1989. 33:175–82.
20). Clinical and Laboratory Standards Institute (CLSI). Performance standard for antimicrobial susceptibility testing; 15th informational supplement. M100-S15. Clinical and Laboratory Standards Institute, Wayne, PA. 2005.
21). Hanssen AM., Fossum A., Mikalsen J., Halvorsen DS., Bukholm G., Sollid J. Dissemination of Community-acquired methicillin-resistant Staphylococcus aureus clones in Northern Norway: sequence type 8 and 80 predominate. J Clin Microbiol. 2005. 43:2118–24.
22). Ko KS., Lee JY., Suh JY., Oh WS., Peck KR., Lee NY., Song JH. Distribution of major genotypes among methicillin-resistant Staphylococcus aureus clones in Asian countries. J Clin Microbiol. 2005. 43:421–6.
23). Kim JS., Song W., Kim HS., Cho HC., Lee KM., Choi MS., Kim EC. Association between the methicillin resistance of clinical isolates of Staphylococcus aureus, their staphylococcal cassette chromosome mec (SCCmec) subtype classification, and their toxin gene profiles. Diagn Microbiol Infect Dis. 2006. 56:289–95.
24). Ma XX., Ito T., Chongtrakool P., Hiramatsu K. Predominance of clones carrying Panton-Valentine Leukocidin genes among methicillin-resistant Staphylococcus aureus strains isolated in Japanese hospitals from 1979 to 1985. J Clin Microbiol. 2006. 44:4515–27.
25). Zaraket H., Otsuka T., Saito K., Dohmae S., Takano T., Higuchi W., Ohkubo T., Ozaki K., Takano M., Reva I., Baranovich T., Yamamoto T. Molecular characterization of methicillin-resistant Staphylococcus aureus in hospitals in Niigata, Japan: Divergence and transmission. Microbiol Immunol. 2007. 51:171–6.
26). File TM Jr. Impact of community-acquired methicillin-resistant Staphylococcus aureus in the hospital setting. Cleve Clin J Med 74, suppl. 2007. 4:S6–11.
27). Ryu JH., Lee HK. Coagulase typing and antibiotic resistance of methicillin resistant Staphylococcus aureus (MRSA) isolated from patients in Pusan. Kor J Microbiol. 2000. 36:216–20.
28). Hwang SM., Kim TU. Changes in coagulase serotype of Staphylococcus aureus isolates in Busan, 1994-2005. Korean J Microbiol. 2007. 43:346–50.
29). Enright MC., Robinson DA., Randle G., Feil EJ., Grundmann H., Spratt BG. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc Natl Acad Sci USA. 2002. 99:7687–92.
Figure 1.
Detection of mecA genes from MRSA isolates by PCR. Lane 1: S. aureus (ATCC25923), Lanes 2 to 3: MRSA isolates, Lanes 4 to 5: MSSA isolates, M, molecular markers, 100 bp DNA ladder (Bioneer).
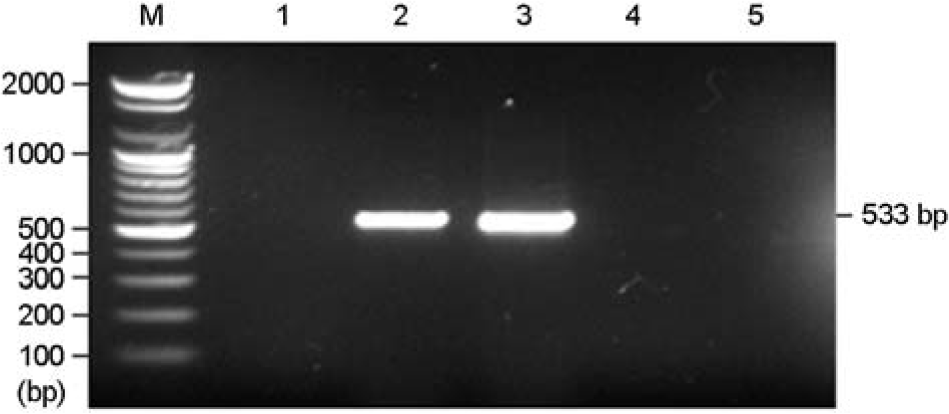
Figure 2.
SCCmec types identified by multiplex PCR. Lanes 1, 4, 7, 10 (loci A and B primers): lanes 2, 5, 8, 11 (loci C and D): lanes 3, 6, 9, 12 (loci E to H): SCCmec type II, lanes 1 to 3 (284 bp, 209 bp, 342 bp): type III, lanes 4 to 6 (209 bp, 243 bp, 414 bp): type IIIA, lanes 7 to 9 (209 bp, 243 bp, 303 bp, 414 bp): type IV, lanes 10 to 12 (342 bp). M, molecular markers, 100 bp DNA ladder (Bioneer).
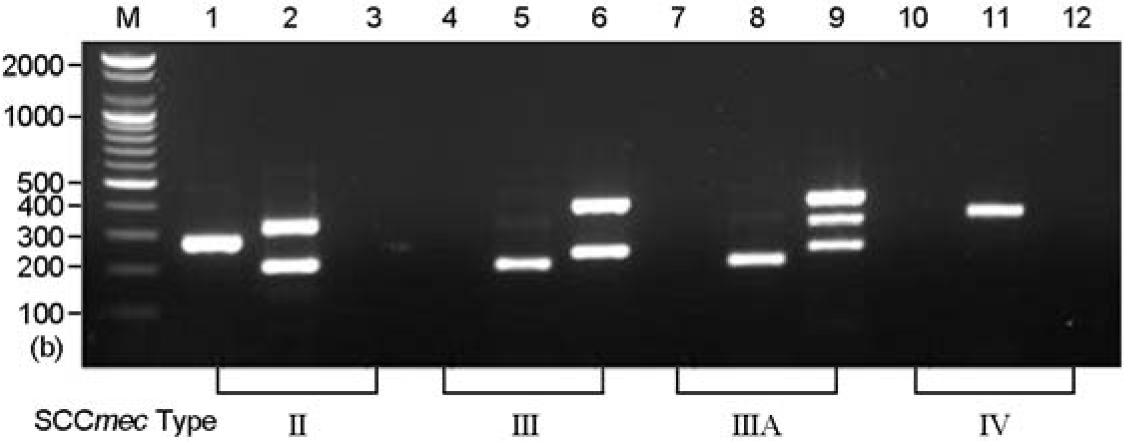
Table 1.
Primers and predicted sizes of PCR products for SCCmec typing
Table 2.
SCCmec types of MRSA strains isolated from clinical sources
Table 3.
Distribution of coagulase serotypes of MRSA strains
Table 4.
Correlation between SCCmec type and coagulase serotype of MRSA
| SCCmec type | No. of strains | Coagulase serotype | |||||
|---|---|---|---|---|---|---|---|
| II | III | IV | V | VII | ND | ||
| II | 60 | 59 | 0 | 0 | 0 | 0 | 1 |
| III | 14 | 0 | 0 | 14 | 0 | 0 | 0 |
| IIIA | 9 | 0 | 0 | 9 | 0 | 0 | 0 |
| IV | 23 | 0 | 1 | 0 | 20 | 1 | 1 |
| ND | 2 | 0 | 0 | 0 | 0 | 2 | 0 |
| Total | 108 | 59 | 1 | 23 | 20 | 3 | 2 |
Table 5.
Antimicrobial susceptibility profile of MRSA strains according to SCCmec




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download