Abstract
In patients with inflammatory bowel disease (IBD), cytomegalovirus (CMV) infections could aggravate the course of IBD but it is difficult to distinguish CMV infection from IBD exacerbation endoscopically. Usually, CMV tends to localize to the colon and other organic involvements were reported very rare in the IBD patients. Herein, we report a case that CMV gastric ulcer complicated with pyloric obstruction in a patient with ulcerative colitis during ganciclovir therapy, which was resolved by surgical gastrojejunostomy with review of literature.
Cytomegalovirus (CMV) infections are usually asymptomatic in most immunocompetent individuals. However, in the case of immunocompromised patients with malignant tumors, organ transplantations, and immunosuppressant drugs, it can cause severe opportunistic infections, such as hepatitis, gastrointestinal disease, retinitis, and encephalitis.1
The gastrointestinal involvement of CMV infection in immunosuppressed patients can occur in any part of the gastrointestinal tract, manifested with esophagitis, gastritis, intestinal ulcers, and focal and diffuse colitis. In addition, CMV infections have been known to complicate the course of inflammatory bowel disease (IBD), especially ulcerative colitis (UC).2 It has been reported that the involvement of CMV infection simultaneously in the stomach and colon is extremely rare in UC. Therefore, we report, with literature review, a case in which CMV gastric ulcer was complicated into complete gastric outlet obstruction in the middle of antiviral therapy in a patient with ulcerative colitis, which was superinfected by CMV infection.
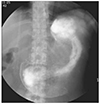
A 36-year-old female patient was admitted presenting watery diarrhea and epigastric pain. Diarrhea developed remotely 6 months ago, which exacerbated 10 days prior to admission with epigastric pain. She had gotten Cesarean section one month ago, and no other medical history was reported. On physical examination, abdominal tenderness, without rebound tenderness, was observed in the epigastric area. Laboratory results revealed leukocytosis, mild anemia, and hypoalbuminemia: white blood cell 25,500/mm3 (neutrophil 70%, lymphocyte 20%, eosinophil 5%), hemoglobin 11.2 g/dL, hematocrit 33.4%, platelet 481,000/mm3, aspartate transaminase/alanine transaminase 7/12 IU/L, Total protein/albumin 5.3/2.0 mg/dL, total bilirubin 0.2 mg/dL, alkaline phosphatase 102 IU/L. According to an upper endosocopy, a huge ulcerative lesion invading the entire antrum circumferentially was found, with a positive result of rapid urease test. The margin of ulcer was relatively clear, and the base was covered with exudates. Erythematous and friable mucosa with partially multiple mucosal defects were discovered across the entire colon, according to a colonoscopy for the evaluation of diarreha (Fig. 1). She was diagnosed endoscopically as active ulcerative colitis, accompanied by gastric ulcer. We started with steroid pulse therapy (intravenous methy-prednisolone 20 mg daily) for ulcerative colitis and proton pump inhibitor for gastric ulcer. In spite of steroid and proton pump inhibitor treatment, her symptoms did not improve. On the 8th day, according to her pathologic report, crypt abscess and inflammatory cell infiltration were found in the colonic mucosa, consistent with ulcerative colitis, and eosinophilic intranuclear inclusion body with acute inflammatory change was also noted, implying CMV infection (Fig. 2). To manage CMW infection, anti-viral agent, such as ganciclovir, was started and steroid treatment was tapered out. After the treatment using antiviral agent, her symptoms were greatly improved; however, on the 10th day of anti-viral therapy (the 44th day from admission), she complained of continuous vomiting. In a follow-up upper endoscopy, a large amount of fluid collections and pyloric stricture with healing ulcer were seen in the stomach, and as a result, the scope was not able to pass through. In the upper gastrointestinal series on the 45th day since admission, the dye did not pass through the pylorus, suggesting a complete pyloric obstruction (Fig. 3). Consequently, endoscopic balloon dilatation was attempted, but failed, and surgical gastrojejunostomy was performed on the 47th day post-hospitalization. After the operation, vomiting was relieved. Then, she was discharged and she did not relapse during the follow-up period.
The pathophysiology of gastrointestinal ulcer by CMV infection is believe to be associated with the invasion of the endothelium of the vessels in the submucosa and causes vasculitis. Fibrin clots are formed, blocking direct blood flow and causing ischemia, ultimately leading to the occurrence of mucosal ulcers.3 According to the endoscopic findings, erythema, erosion, ulcer, bleeding, mucosal hypertrophy, and pseudo-polyps can be shown solitarily or multiply. However, it is known that there is no typical endoscopic finding of CMV infection in the gastrointestinal tract.4 Therefore, if clinically suspected, it is necessary to perform a biopsy in the ulcer base, rather than the margin since CMV mainly invades the endothelium of the submucosal layer, fibroblasts, smooth muscle cells, and glandular cells of the gastrointestinal tract.5
In IBD patients, CMV infections could aggravate the course of IBD. The incidence of CMV infections in IBD patients receiving immunosuppressive therapy has been reported to be between 15.8 and 34%.6 Moreover, it is difficult to endoscopically distinguish CMV infections from IBD exacerbation. Hence, we should suspect CMV infection in patients with IBD who were refractory to immunosuppressive drugs, such as steroid. Additionally, CMV usually tends to localize around the colon, with a very rare occurrence of dissemination to other organs in patients with IBD.7 In our case, CMV simultaneously invaded the stomach and colon in a UC patient, as confirmed by the pathological report. Therefore, CMV infection can be suspected if there were no other accompanying symptoms except colon-related ones in IBD patients.
During the treatment of CMV infection with anti-viral agents, complications of complete gastric outlet obstruction occurred as a result of gastric ulcer healing process, which was resolved by a surgical gastrojejunostomy. In general, gastric ulcer disease may be the underlying cause in less than 5 to 8 percent of patients with gastric outlet obstruction.8 Mostly gastric ulcer disease is associated with duodenal or pyloric channel ulceration. In our case, gastric ulcer was located on the pyloric channel and antrum, circumferentially and the patient was a high risk group for gastric outlet obstruction. Therefore, to prevent gastric outlet obstruction, it would be better to place a temporary stent in the pylorus before starting any anti-viral and anti-ulcer medications.9 In our case, temporary stent insertion was not possible due to complete obstruction.
In conclusion, it is important to suspect the possibility of CMV infection in IBD patients who were unresponsive to immunosuppressant drugs with other accompanying symptoms or signs that might suggest the involvement of other organs.
Figures and Tables
Fig. 1
Endoscopic findings. (A) Endoscopic finding of the gastric lesions. It shows a huge, deepencirculating ulcerative lesion on the antrum, covered with white exudate, with relatively clear margin and even base. (B) Colonoscopic finding. It shows erythematous and friable mucosa from the cecum to the rectum with multiple mucosal defects.
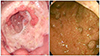
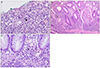
Fig. 2
Pathologic findings. (A) Typical crypt abscess, showing collection of PMNs in the crypts (arrow) is noted in colonic mucosa (colon, H&E, ×100). (B) Typical eosinophilic inclusion bodys are noted in the nucleus with halo (arrows) of the CMV infected cell (colon, H&E, ×400). (C) Eosinophilic inclusion body (arrow) is noted in the nucleus of the endothelial cell in the gastric mucosa (stomach, H&E, ×400). PMNs, polymorphonuclear; CMV, cytomegalovirus.
References
1. Weller TH. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. II. N Eng J Med. 1971; 285:267–274.
2. Hannant KL, Rotterdam HZ, Bell ET, Tapper ML. Cytomegalovirus infection of the alimentary tract: a clinicopathological correlation. Am J Gastroenterol. 1986; 81:944–950.
3. Lee G, Kim NI, Gu JT, Suh JI, Yang CH, Lee CW. A case of cytomegalovirus colitis in an immunocompetent adult. Korean J Gastroenterol. 2000; 35:649–653.
4. Chon SY, Lim YJ, Kim MY, et al. A case of cytomegalovirus (CMV) colitis in a patient after splenectomy. Korean J Gastrointest Endosc. 2003; 26:158–162.
5. Foucar E, Mukai K, Foucar K, Sutherland DE, Van Buren CT. Colon ulceration in lethal cytomegalovirus infection. Am J Clin Pathol. 1981; 76:788–801.
6. Papadakis KA, Tung JK, Binder SW, et al. Outcome of cytomegalovirus infections in patients with inflammatory bowel disease. Am J Gastroenterol. 2001; 96:2137–2142.
7. Ye BD, Kim SG, Kim JS, et al. A case of cytomegalovirus-associated multiple gastric ulcers in ulcerative colitis. Int J Colorectal Dis. 2007; 22:1419–1420.
8. Gisbert JP, Pajares JM. Review article: helicobacter pylori infection and gastric outlet obstruction-prevalence of the infection and role of antimicrobial treatment. Aliment Pharmacol Ther. 2002; 16:1203–1208.
9. Dormann AJ, Deppe H, Wigginghaus B. Self-expanding metallic stents for continuous dilataion of benign stenoses in gastrointestinal tract - first results of long-term follow-up in interim stent application in pyloric and colonic obstructions. Z Gastroenterol. 2001; 39:957–960.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download