Abstract
Background/Aims
Gemcitabine-based chemotherapy has been used as a standard treatment in patients with unresectable pancreatic cancer. However, the clinical outcomes of this regimen are still unsatisfactory in prolonging survival. We retrospectively analyzed clinical characteristics of patients with advanced pancreatic cancers who received gemcitabine-based chemotherapy and showed long-term survival.
Methods
We enrolled 49 patients who underwent treatment with more than three cycles of gemcitabine-based chemotherapy. Long-term survivor was defined as patient who has survived more than 12 months after diagnosis. The clinical characteristics were analyzed to compare the differences between long-term and short-term survivors. Univariate or multivariate analyses were performed to identify prognostic factors associated with chemo-responses.
Results
Twenty patients (41%) survived more than 12 months. Long-term survivors had smaller tumor size (OR 2.190, p=0.049, 95% CI 1.005-4.773) and higher serum BUN level (OR 0.833, p=0.039, 95% CI 0.701-0.990) compared to short-term survivors. Overall median and progression-free survivals were 11 and 4 months, respectively. Presence of distant metastasis (hazard ratio 1.441, p=0.035, 95% CI 1.002-2.908) was a significant independent predictor of progression-free survival. Tumor size (hazard ratio 1.534, p=0.004, 95% CI 1.150-2.045) was associated with overall survival.
Conclusions
Gemcitabine chemotherapy may be more effective and allow longer survivals in patients with clinical characters of smaller tumor size and normal serum BUN level at diagnosis. We suggest a well-designed large controlled study to evaluate the prognostic factors such as clinical characteristics and molecular biological features in patients with advanced pancreatic cancers who receive gemcitabine-based chemotherapy.
References
1. Annual report on the cause of death statistics. [Internet]. Daejeon: Statistics Korea [cited 2014 Sep 11]. Available from:. http://kostat.go.kr/portal/korea/kor_ko/5/2/index.board?bmode=read&aSeq=308628=read&aSeq=308628.
2. Fernández-del Castillo C, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg. 1995; 130:295–299.

3. DiMagno EP, Reber HA, Tempero MA. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. American Gastroenterological Association. Gastroenterology. 1999; 117:1464–1484.
4. Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997; 15:2403–2413.

5. Jo JH, Chung MJ, Park JY, et al. Clinical characteristics of long-term survivors of inoperable pancreatic cancer: an 8-year cohort analysis in Korea. Pancreas. 2014; 43:1022–1031.
6. Kim YJ, Seo DW, Pack KM, et al. The prognostic factors of pancreatic cancer can be different according to clinical stages. Korean J Gastroenterol. 2008; 51:181–189.
7. Moore MJ, Goldstein D, Hamm J, et al. National Cancer Institute of Canada Clinical Trials Group. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007; 25:1960–1966.

8. Herrmann R, Bodoky G, Ruhstaller T, et al. Swiss Group for Clinical Cancer Research; Central European Cooperative Oncology Group. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007; 25:2212–2217.

9. Carmichael J, Fink U, Russell RC, et al. Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. 1996; 73:101–105.

10. Casper ES, Green MR, Kelsen DP, et al. Phase II trial of gemcitabine (2,2'-difluorodeoxycytidine) in patients with adenocarcinoma of the pancreas. Invest New Drugs. 1994; 12:29–34.
11. Park JK, Yoon YB, Kim YT, Ryu JK, Yoon WJ, Lee SH. Survival and prognostic factors of unresectable pancreatic cancer. J Clin Gastroenterol. 2008; 42:86–91.

12. Ikeda M, Okada S, Tokuuye K, Ueno H, Okusaka T. Prognostic factors in patients with locally advanced pancreatic carcinoma receiving chemoradiotherapy. Cancer. 2001; 91:490–495.

13. Krishnan S, Rana V, Janjan NA, et al. Prognostic factors in patients with unresectable locally advanced pancreatic adenocarcinoma treated with chemoradiation. Cancer. 2006; 107:2589–2596.

14. Papadoniou N, Kosmas C, Gennatas K, et al. Prognostic factors in patients with locally advanced (unresectable) or metastatic pancreatic adenocarcinoma: a retrospective analysis. Anticancer Res. 2008; 28:543–549.
15. Weber A, Kehl V, Mittermeyer T, et al. Prognostic factors for survival in patients with unresectable pancreatic cancer. Pancreas. 2010; 39:1247–1253.

16. Yi JH, Lee J, Park SH, et al. A prognostic model to predict clinical outcomes with first-line gemcitabine-based chemotherapy in advanced pancreatic cancer. Oncology. 2011; 80:175–180.

17. Furukawa K, Uwagawa T, Iwase R, et al. Prognostic factors of unresectable pancreatic cancer treated with nafamostat mesilate combined with gemcitabine chemotherapy. Anticancer Res. 2012; 32:5121–5126.
Fig. 1.
Consort algorithm of patient selection. We enrolled 49 patients who underwent more than three cycles of gemcitabine-based chemotherapy without radiation therapy and received regular follow up. CTx, chemotheraphy.
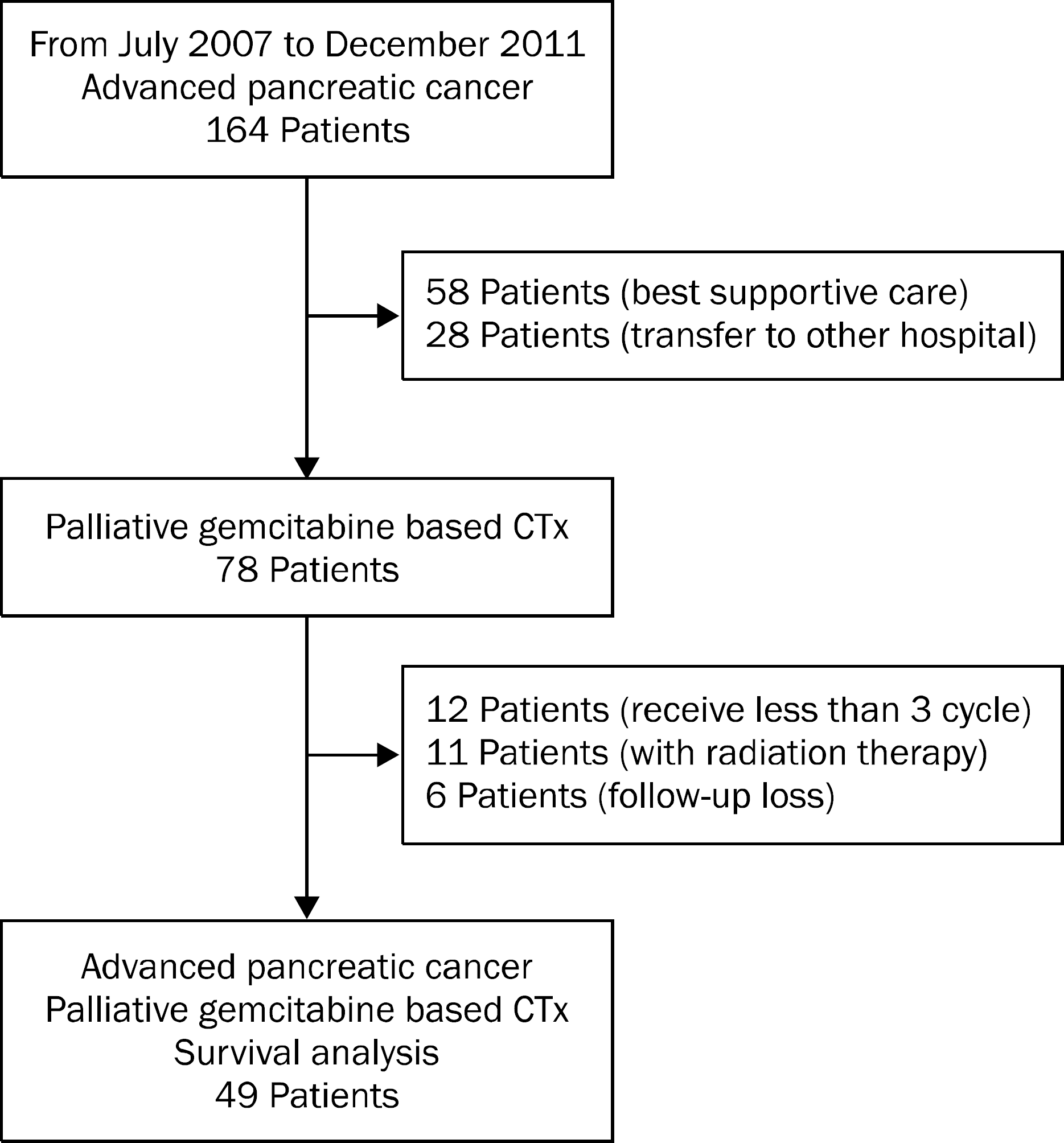
Fig. 2.
Progression free survival (PFS) curves according to the presence of distant metastasis (A) and overall survival (OS) curves according to the tumor size (B) in patients with advanced pancreatic cancers who received gemcitabine-based chemotherapy. Distant metastasis (hazard ratio 1.441, p=0.035, 95% CI 1.002-2.908) and tumor size (hazard ratio 1.534, p=0.004, 95% CI 1.150-2.045) were related to PFS and OS, respectively.
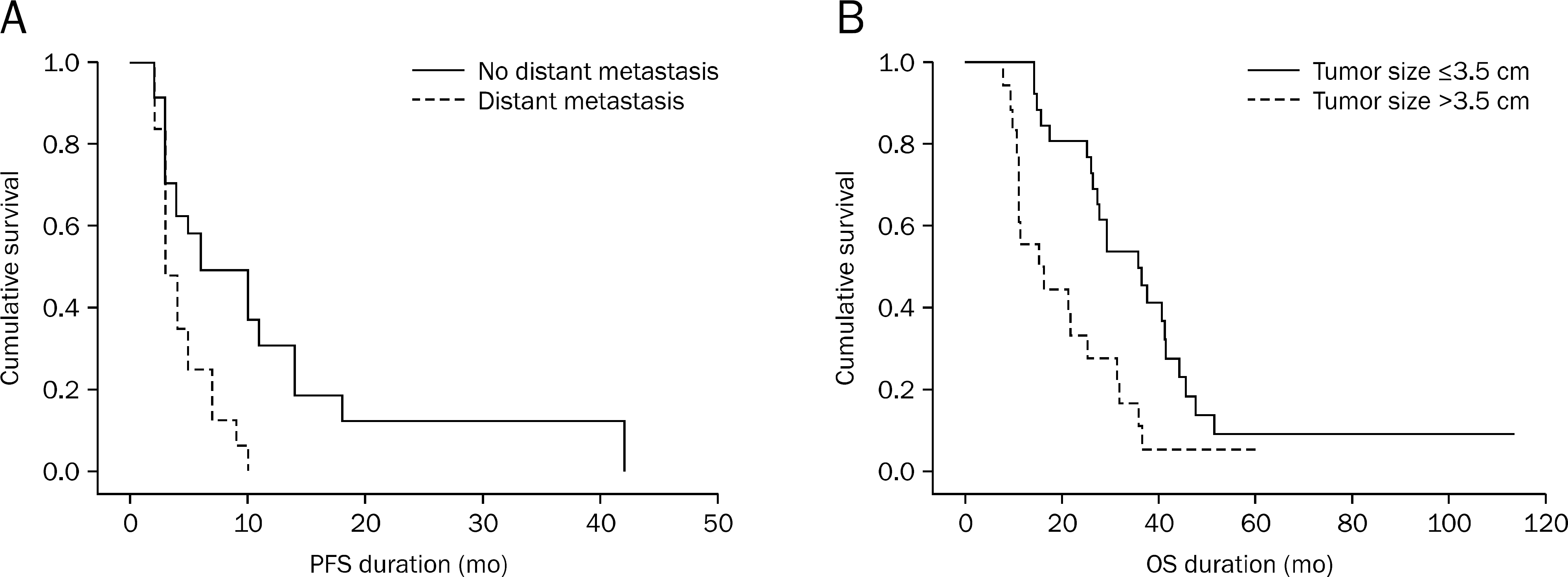
Table 1.
Baseline Characteristics of Patients with Pancreatic Cancer
Table 2.
Factors Influencing Survival at One Year on Univariate Analysis
Table 3.
Factors Influencing Overall Survival More than One Year on Multivariate Logistic Regression Analysis
Table 4.
Factors Influencing Disease Progression Free Survival
Table 5.
Factors Influencing Overall Survival




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download