Abstract
Endoscopic resection (ER) is accepted as a treatment option for early gastric cancer in patients with negligible risk of lymph node metastasis. Determination of histologic differentiation of adenocarcinoma based on pretreatment endoscopic biopsy is critical in deciding the treatment strategy of ER versus surgical resection. However, discrepancies are frequent between pretreatment biopsies and ER specimens, which may result in an additional gastrectomy after ER. In this context, a review on possible factors contributing to the diagnostic discrepancy in the histologic difference between the pretreatment biopsy and ER is necessary. Two major factors are significantly associated with this discrepancy: pathologic characteristics of the tumor itself, i.e. histologic heterogeneity (tumor factor), and diagnostic procedure performed by endoscopists or pathologists (human factor). In this review, we focus on pathologic report of pretreatment biopsy specimens and its clinical significance.
References
1. Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000; 3:219–225.

2. Lee JH, Kim JG, Jung HK, et al. Committee on Stomach Cancer Guideline Development. Synopsis on clinical practice guideline of gastric cancer in Korea: an evidence-based approach. Korean J Gastroenterol. 2014; 63:66–81.

3. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14:113–123.
4. Shim CN, Kim H, Kim DW, et al. Clinicopathologic factors and outcomes of histologic discrepancy between differentiated and undifferentiated types after endoscopic resection of early gastric cancer. Surg Endosc. 2014; 28:2097–2105.

5. Takao M, Kakushima N, Takizawa K, et al. Discrepancies in histologic diagnoses of early gastric cancer between biopsy and endoscopic mucosal resection specimens. Gastric Cancer. 2012; 15:91–96.

6. Lee IS, Park YS, Lee JH, et al. Pathologic discordance of differentiation between endoscopic biopsy and postoperative specimen in mucosal gastric adenocarcinomas. Ann Surg Oncol. 2013; 20:4231–4237.

7. Kim WH, Park CK, Kim YB, et al. A standardized pathology report for gastric cancer. Korean J Pathol. 2005; 39:106–113.
8. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14:101–112.
9. Lauwers GY, Carneiro F, Graham DY, et al. World Health Organization classification of tumours: WHO classification of tumours of the digestive system. Lyon: International Agency for Research on Cancer;2010. p. 53.
10. Mita T, Shimoda T. Risk factors for lymph node metastasis of submucosal invasive differentiated type gastric carcinoma: clinical significance of histological heterogeneity. J Gastroenterol. 2001; 36:661–668.

11. Hanaoka N, Tanabe S, Mikami T, Okayasu I, Saigenji K. Mixed- histologic-type submucosal invasive gastric cancer as a risk factor for lymph node metastasis: feasibility of endoscopic submucosal dissection. Endoscopy. 2009; 41:427–432.
12. Nakata K, Nagai E, Miyasaka Y, et al. The risk of lymph node metastasis in mucosal gastric carcinoma: especially for a mixture of differentiated and undifferentiated adenocarcinoma. Hepatogastroenterology. 2012; 59:1855–1858.
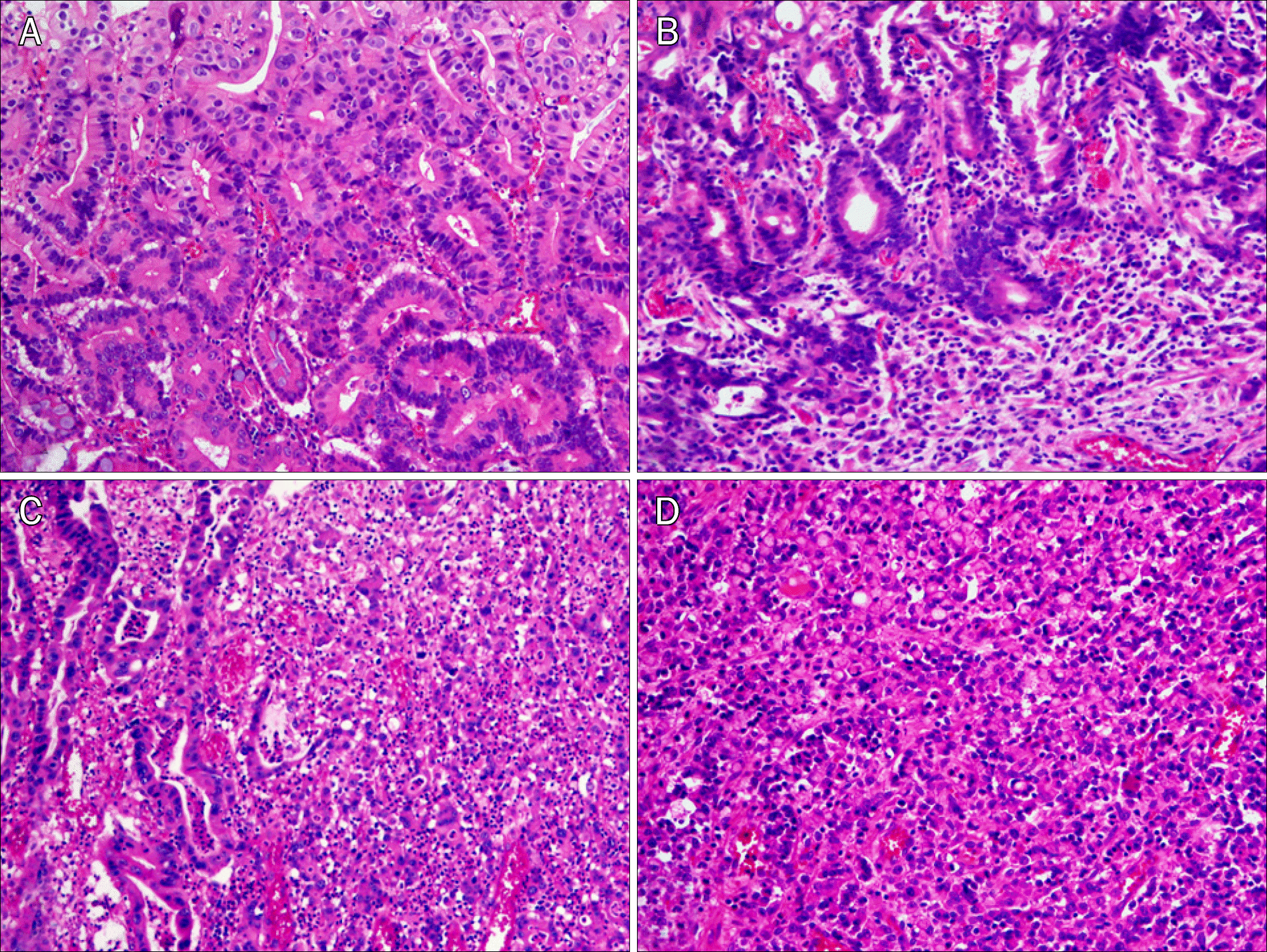
Fig. 1.
Representative microphotographs (H&E, ×200). (A) Pure differentiated type consisting of well differentiated tubular adenocarcinoma without any undifferentiated type components. (B) Differentiated-predominant mixed type showing a predominance of well differentiated tubular adenocarcinoma with a small area of poorly cohesive carcinoma in the deeper portion. (C) Undifferentiated-predominant mixed type showing a predominance of poorly cohesive carcinoma with less than 50% of well to moderately differentiated adenocarcinoma. (D) Pure undifferentiated type consisting of undifferentiated type components only.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download