1. Wu G, Bazer FW, Wallace JM, Spencer TE. Board-invited review: intrauterine growth retardation: implications for the animal sciences. J Anim Sci. 2006; 84:2316–2337. PMID:
16908634.
2. Valsamakis G, Kanaka-Gantenbein C, Malamitsi-Puchner A, Mastorakos G. Causes of intrauterine growth restriction and the postnatal development of the metabolic syndrome. Ann N Y Acad Sci. 2006; 1092:138–147. PMID:
17308140.

3. Hock MB, Kralli A. Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol. 2009; 71:177–203. PMID:
19575678.

4. Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005; 120:483–495. PMID:
15734681.

5. Park KS, Kim SK, Kim MS, Cho EY, Lee JH, Lee KU, Pak YK, Lee HK. Fetal and early postnatal protein malnutrition cause long-term changes in rat liver and muscle mitochondria. J Nutr. 2003; 133:3085–3090. PMID:
14519789.

6. Reusens B, Sparre T, Kalbe L, Bouckenooghe T, Theys N, Kruhøffer M, Ørntoft TF, Nerup J, Remacle C. The intrauterine metabolic environment modulates the gene expression pattern in fetal rat islets: prevention by maternal taurine supplementation. Diabetologia. 2008; 51:836–845. PMID:
18311556.

7. Zhang H, Li Y, Hou X, Zhang L, Wang T. Medium-chain TAG improve energy metabolism and mitochondrial biogenesis in the liver of intra-uterine growth-retarded and normal-birth-weight weanling piglets. Br J Nutr. 2016; 115:1521–1530. PMID:
26960981.

8. Peterside IE, Selak MA, Simmons RA. Impaired oxidative phosphorylation in hepatic mitochondria in growth-retarded rats. Am J Physiol Endocrinol Metab. 2003; 285:E1258–E1266. PMID:
14607783.

9. Lane RH, Flozak AS, Ogata ES, Bell GI, Simmons RA. Altered hepatic gene expression of enzymes involved in energy metabolism in the growth-retarded fetal rat. Pediatr Res. 1996; 39:390–394. PMID:
8929856.

10. Li F, Yin Y, Tan B, Kong X, Wu G. Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids. 2011; 41:1185–1193. PMID:
21773813.

11. Wu G. Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. J Anim Sci Biotechnol. 2014; 5:34. PMID:
24999386.

12. Wilson J, Wilson GJ. Contemporary issues in protein requirements and consumption for resistance trained athletes. J Int Soc Sports Nutr. 2006; 3:7–27. PMID:
18500966.

13. Garlick PJ. The role of leucine in the regulation of protein metabolism. J Nutr. 2005; 135:1553S–1556S. PMID:
15930468.

14. Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR. Bidirectional modulation of insulin action by amino acids. J Clin Invest. 1998; 101:1519–1529. PMID:
9525995.

15. Layman DK. The role of leucine in weight loss diets and glucose homeostasis. J Nutr. 2003; 133:261S–267S. PMID:
12514305.

16. Li H, Xu M, Lee J, He C, Xie Z. Leucine supplementation increases SIRT1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. Am J Physiol Endocrinol Metab. 2012; 303:E1234–E1244. PMID:
22967499.

17. Vaughan RA, Garcia-Smith R, Gannon NP, Bisoffi M, Trujillo KA, Conn CA. Leucine treatment enhances oxidative capacity through complete carbohydrate oxidation and increased mitochondrial density in skeletal muscle cells. Amino Acids. 2013; 45:901–911. PMID:
23812674.

18. Sun X, Zemel MB. Leucine modulation of mitochondrial mass and oxygen consumption in skeletal muscle cells and adipocytes. Nutr Metab (Lond). 2009; 6:26. PMID:
19500359.

19. Xu W, Bai K, He J, Su W, Dong L, Zhang L, Wang T. Leucine improves growth performance of intrauterine growth retardation piglets by modifying gene and protein expression related to protein synthesis. Nutrition. 2016; 32:114–121. PMID:
26454700.

20. Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol. 2012; 20:50–57. PMID:
22153753.

21. Institute of Laboratory Animal Resources (US). Guide for the Care and Use of Laboratory Animals. 7th ed. Washington, D.C.: National Academy Press;1996.
22. Wang Y, Zhang L, Zhou G, Liao Z, Ahmad H, Liu W, Wang T. Dietary L-arginine supplementation improves the intestinal development through increasing mucosal Akt and mammalian target of rapamycin signals in intra-uterine growth retarded piglets. Br J Nutr. 2012; 108:1371–1381. PMID:
22217383.

23. National Research Council (US), Committee on Nutrient Requirements of Swine. National Research Council (US), Board on Agriculture and Natural Resources. Nutrient Requirements of Swine. 11th rev. ed. Washington, D.C.: National Academy Press;2012.
24. Yi D, Hou Y, Wang L, Ding B, Yang Z, Li J, Long M, Liu Y, Wu G. Dietary N-acetylcysteine supplementation alleviates liver injury in lipopolysaccharide-challenged piglets. Br J Nutr. 2014; 111:46–54. PMID:
23829996.
25. Atkinson DE. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968; 7:4030–4034. PMID:
4972613.
26. Weinbach EC. A procedure for isolating stable mitochondria from rat liver and kidney. Anal Biochem. 1961; 2:335–343. PMID:
13783868.

27. Nemeria N, Yan Y, Zhang Z, Brown AM, Arjunan P, Furey W, Guest JR, Jordan F. Inhibition of the Escherichia coli pyruvate dehydrogenase complex E1 subunit and its tyrosine 177 variants by thiamin 2-thiazolone and thiamin 2-thiothiazolone diphosphates. Evidence for reversible tight-binding inhibition. J Biol Chem. 2001; 276:45969–45978. PMID:
11583990.
28. Shepherd D, Garland PB. The kinetic properties of citrate synthase from rat liver mitochondria. Biochem J. 1969; 114:597–610. PMID:
5820645.

29. Meixner-Monori B, Kubicek CP, Harrer W, Schreferl G, Rohr M. NADP-specific isocitrate dehydrogenase from the citric acid-accumulating fungus Aspergillus niger. Biochem J. 1986; 236:549–557. PMID:
3753466.

30. Lai JC, Cooper AJ. Brain alpha-ketoglutarate dehydrogenase complex: kinetic properties, regional distribution, and effects of inhibitors. J Neurochem. 1986; 47:1376–1386. PMID:
3760866.
31. Mehler AH, Kornberg A, Grisolia S, Ochoa S. The enzymatic mechanism of oxidation-reductions between malate or isocitrate and pyruvate. J Biol Chem. 1948; 174:961–977. PMID:
18871256.

32. Ragan CI. Structure and function of an archetypal respiratory chain complex: NADH-ubiquinone reductase. Biochem Soc Trans. 1990; 18:515–516. PMID:
2125941.

33. Medja F, Allouche S, Frachon P, Jardel C, Malgat M, Mousson de Camaret B, Slama A, Lunardi J, Mazat JP, Lombès A. Development and implementation of standardized respiratory chain spectrophotometric assays for clinical diagnosis. Mitochondrion. 2009; 9:331–339. PMID:
19439198.

34. Krähenbühl S, Talos C, Wiesmann U, Hoppel CL. Development and evaluation of a spectrophotometric assay for complex III in isolated mitochondria, tissues and fibroblasts from rats and humans. Clin Chim Acta. 1994; 230:177–187. PMID:
7834868.

35. Wharton DC, Tzagoloff A. Cytochrome oxidase from beef heart mitochondria. Methods Enzymol. 1967; 10:245–250.
36. Taussky HH, Shorr E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953; 202:675–685. PMID:
13061491.

37. Liu J, Yao Y, Yu B, Mao X, Huang Z, Chen D. Effect of folic acid supplementation on hepatic antioxidant function and mitochondrial-related gene expression in weanling intrauterine growth retarded piglets. Livest Sci. 2012; 146:123–132.

38. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001; 29:e45. PMID:
11328886.

39. D'Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, Caliaro F, Corsetti G, Bottinelli R, Carruba MO, Valerio A, Nisoli E. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010; 12:362–372. PMID:
20889128.
40. Ogata ES, Swanson SL, Collins JW Jr, Finley SL. Intrauterine growth retardation: altered hepatic energy and redox states in the fetal rat. Pediatr Res. 1990; 27:56–63. PMID:
2296473.

41. Lim S, Cho YM, Park KS, Lee HK. Persistent organic pollutants, mitochondrial dysfunction, and metabolic syndrome. Ann N Y Acad Sci. 2010; 1201:166–176. PMID:
20649553.

42. Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003; 24:78–90. PMID:
12588810.
43. Liu J, Yu B, Mao X, He J, Yu J, Zheng P, Huang Z, Chen D. Effects of intrauterine growth retardation and maternal folic acid supplementation on hepatic mitochondrial function and gene expression in piglets. Arch Anim Nutr. 2012; 66:357–371. PMID:
22889112.

44. Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012; 13:225–238. PMID:
22395773.

45. Virbasius CA, Virbasius JV, Scarpulla RC. NRF-1, an activator involved in nuclear-mitochondrial interactions, utilizes a new DNA-binding domain conserved in a family of developmental regulators. Genes Dev. 1993; 7:2431–2445. PMID:
8253388.

46. Ekstrand MI, Falkenberg M, Rantanen A, Park CB, Gaspari M, Hultenby K, Rustin P, Gustafsson CM, Larsson NG. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum Mol Genet. 2004; 13:935–944. PMID:
15016765.

47. Maniura-Weber K, Goffart S, Garstka HL, Montoya J, Wiesner RJ. Transient overexpression of mitochondrial transcription factor A (TFAM) is sufficient to stimulate mitochondrial DNA transcription, but not sufficient to increase mtDNA copy number in cultured cells. Nucleic Acids Res. 2004; 32:6015–6027. PMID:
15547250.

48. Simmons RA, Suponitsky-Kroyter I, Selak MA. Progressive accumulation of mitochondrial DNA mutations and decline in mitochondrial function lead to beta-cell failure. J Biol Chem. 2005; 280:28785–28791. PMID:
15946949.
49. Morris TJ, Vickers M, Gluckman P, Gilmour S, Affara N. Transcriptional profiling of rats subjected to gestational undernourishment: implications for the developmental variations in metabolic traits. PLoS One. 2009; 4:e7271. PMID:
19787071.

50. Tatpati LL, Irving BA, Tom A, Bigelow ML, Klaus K, Short KR, Nair KS. The effect of branched chain amino acids on skeletal muscle mitochondrial function in young and elderly adults. J Clin Endocrinol Metab. 2010; 95:894–902. PMID:
20022987.

51. Thomas PJ, Garboczi DN, Pedersen PL. Mutational analysis of the consensus nucleotide binding sequences in the rat liver mitochondrial ATP synthase beta-subunit. J Biol Chem. 1992; 267:20331–20338. PMID:
1400352.

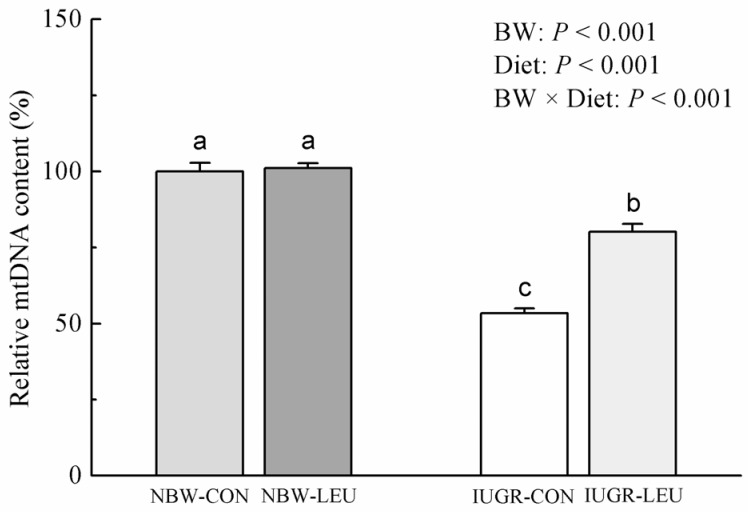
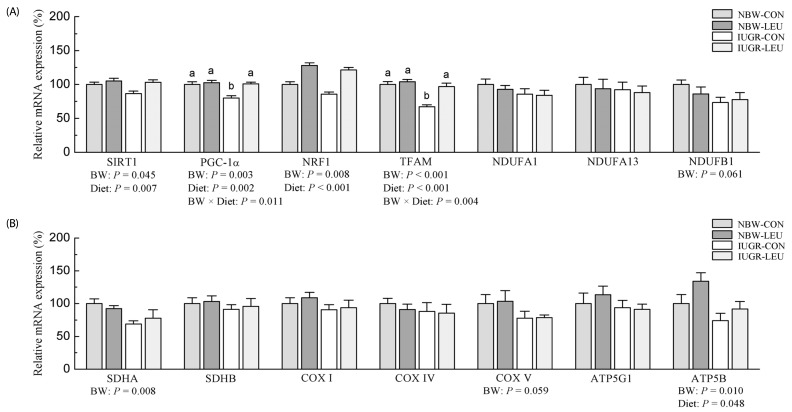
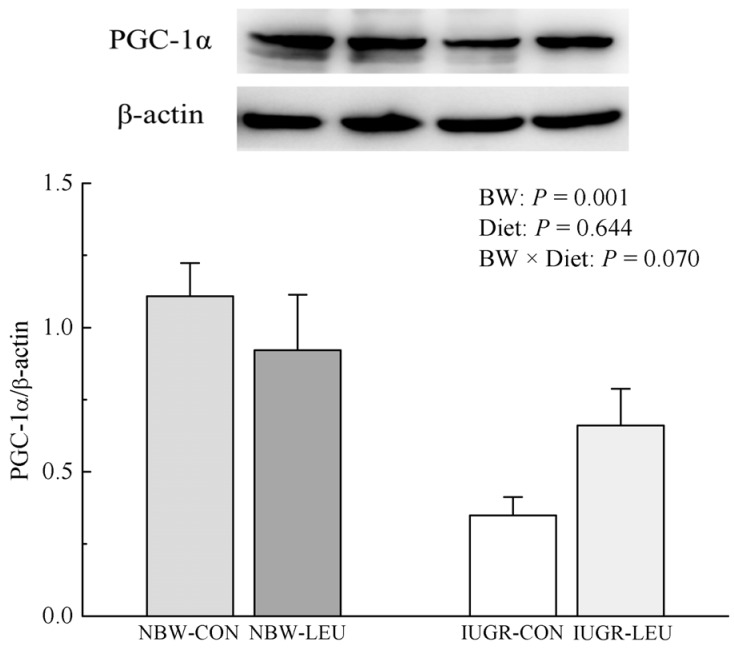
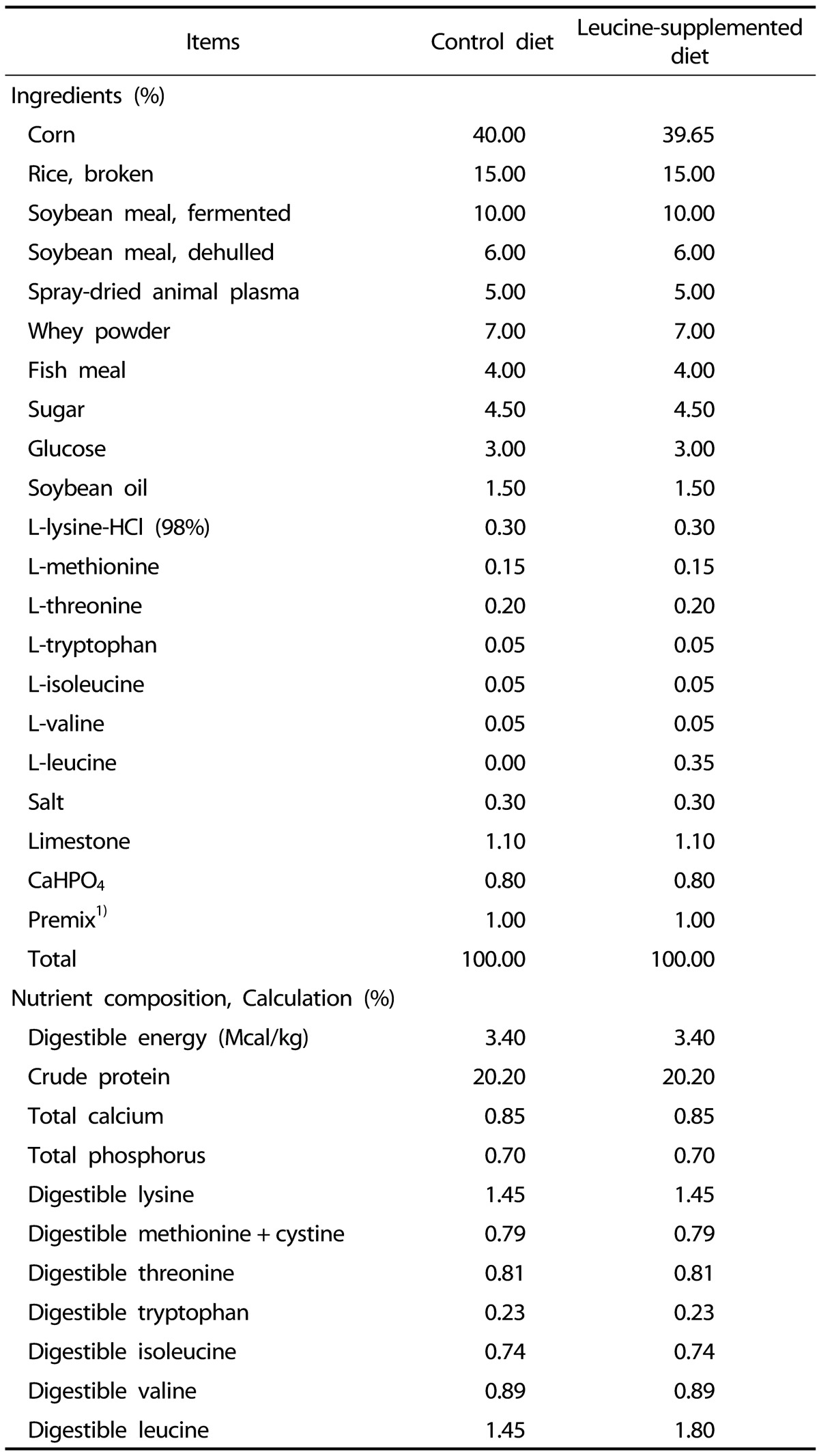





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download