Abstract
Rho iso-alpha acids-rich extract (RIAA) from Humulus lupulus (hops) and proanthocyanidins-rich extracts (PAC) from Acacia nilotica exert anti-inflammatory and anti-diabetic activity in vitro and in vivo. We hypothesized that a combination of these two extracts would exert enhanced effects in vitro on inflammatory markers and insulin signaling, and on nonfasting glucose and insulin in db/db mice. Over 49 tested combinations, RIAA:PAC at 5:1 (6.25 µg/mL) exhibited the greatest reductions in TNFα-stimulated lipolysis and IL-6 release in 3T3-L1 adipocytes, comparable to 5 µg/mL troglitazone. Pretreatment of 3T3-L1 adipocytes with this combination (5 µg/mL) also led to a 3-fold increase in insulin-stimulated glucose uptake that was comparable to 5 µg/mL pioglitazone or 901 µg/mL aspirin. Finally, db/db mice fed with RIAA:PAC at 5:1 (100 mg/kg) for 7 days resulted in 22% decrease in nonfasting glucose and 19% decrease in insulin that was comparable to 0.5 mg/kg rosiglitazone and better than 100 mg/kg metformin. RIAA:PAC mixture may have the potential to be an alternative when conventional therapy is undesirable or ineffective, and future research exploring its long-term clinical application is warranted.
The National Health and Nutrition Examination Survey 1999-2002 reported that nearly 40% of adults in the US were classified as having the metabolic syndrome [1], a disorder associated with obesity, dyslipidemia, atherosclerosis, coronary heart disease, and a predisposition to type 2 diabetes [2,3]. Insulin resistance, a state of reduced responsiveness of a target cell to normal levels of circulating insulin, is a common factor in all of these diseases [4].
In the adipose tissue of mice with high-fat diet-induced obesity, an increased number of macrophages has been observed [5]. The increased macrophage infiltration and cytokine expression has also been observed in the adipose tissue of obese humans [6]. Increased expression of cytokines such as interleukin (IL)-6 and monocyte chemoattractant protein (MCP)-1 in the adipose tissue, as well as tumor necrosis factor (TNF) α released by macrophages, cause inflammation and promote lipolysis and secretion of free fatty acids (FFA) [7,8]. These events result in the disruption of several essential insulin signaling mediators, including insulin receptor substrate (IRS) proteins, the phosphatidylinositol 3-kinase (PI3K)-Akt/protein kinase B (PKB) pathway, glycogen synthase kinase-3 (GSK3) and the Ras-mitogen activated protein kinase (MAPK) pathway in adipose, muscle, liver and other cells [9,10].
Pharmaceutical interventions to increase insulin sensitivity are available. For example, metformin is an insulin sensitizer that suppresses hepatic gluconeogenesis and increases glucose uptake into muscle [11]. It has also been shown to decrease triglycerides and low-density lipoprotein cholesterol [12]. Thiazolidinediones such as rosiglitazone and pioglitazone enhance insulin sensitivity in adipose tissue, liver and skeletal muscle via binding to the nuclear peroxisome proliferator-activated receptor gamma (PPAR γ) [12], although drugs in this class have differential effects on serum lipids [13]. These drugs have been used individually or as combination therapies, but are associated with dangerous adverse effects such as hypoglycemia and weight gain for sulfonylureas and heart failure for thiazolidinediones [14,15]. Further, they do not directly address the underlying causes of insulin resistance.
In search of novel candidates for diabetes management, Babish et al. [16] evaluated a large number of structurally diverse, commercially available phytochemical products for anti-diabetic activity. Results from in vitro evaluations demonstrated that a rho iso-alpha acids-rich extract (RIAA) derived from hops (Humulus lupulus L., (Cannabaceae)), and proanthocyanidins-rich extracts (PAC) from acacia (Acacia nilotica, (Fabaceae)), decreased FFA and IL-6 release and increased adiponectin secretion in TNF-α stimulated mature 3T3-T1 adipocytes. In the db/db mouse model, both RIAA and PAC decreased nonfasting serum glucose by 6.8 and 7.6% (P < 0.01), respectively. PAC also reduced nonfasting serum insulin by 11% (P < 0.05). Another study indicated that RIAA selectively inhibited GSK3β/nuclear factor kappa-B (NF-κB) mediated signaling and reduced lipopolysaccharide (LPS)-stimulated transcription of inflammatory markers [17].
Combinations or formulations of botanical mixtures have been practiced in traditional Chinese Medicine, Ayurvedic medicine and other ethno-phytotherapies for centuries. We hypothesized that a combination of RIAA and PAC may exert enhanced effects on inflammatory markers, insulin signaling, and on nonfasting glucose and insulin. To test this hypothesis, we first determined the optimal ratio of RIAA to PAC for inhibition of FFA release/lipolysis and IL-6 secretion in vitro. A defined ratio of RIAA to PAC was then examined for activity on lipolysis, insulin signaling, and glucose uptake in 3T3-L1 adipocytes. Finally, corresponding to the in vitro experiments, we investigated the effect of the mixture on serum glucose and insulin in obese db/db mice, an in vivo model that exhibited dyslipidemia, insulin resistance, and diabetes.
The RIAA used consisted of a commercial preparation of dried RIAA magnesium salt (Mg-RIAA) provided by John I. Haas (Yakima, WA). This material contained approximately 25% inorganic salts, 5% low-molecular-weight resin, and 68% total rho iso-alpha acids. PAC extract, obtained from KDN-Vita International/Indfrag Ltd. (Hillsborough, NJ), was an aqueous/methanol extract of bark from the A. nilotica tree consisting of approximately 15.9% small molecule catechins and gallates, 28.4% oligomeric proanthocyanidins, and 55.7% polymeric proanthocyanidins, The chemical composition and molecular structures of RIAA (determined by LC-MS and NMR) and PAC (determined by HPLC) have been described in [18]. Heat-inactivated fetal bovine serum (HIFBS), penicillin and streptomycin solution, and Dulbecco's Modification of Eagle's Medium (DMEM) were purchased from Mediatech (Herndon, VA). Pioglitazone was obtained as 45 mg pioglitazone tablets (Actos®) from Takeda Pharmaceuticals (Lincolnshire, IL) and all concentrations were based on the active material although excipients were included. Troglitazone, metformin and rosiglitazone were purchased from Cayman Chemical (Ann Arbor, MI). 2-N-7-(nitrobenz-2-oxa-1,3-diazol-4-yl)amino-2-deoxy-d-glucose (2-NBDG) was obtained from Invitrogen (Carlsbad, CA). Bacterial LPS, murine TNF α and all standard chemicals, unless noted, were obtained from Sigma (St Louis, MO) and were of the highest purity commercially available.
Murine 3T3-L1 preadipocyte cell lines were purchased from American Type Culture Collection (Manassas, VA) and maintained according to instructions from the supplier. Preadipocytes were seeded at an initial density of approximately 3 × 104 cells/cm2 in 24-well plates. Cells were grown for 3 d in a 5% CO2 humidified incubator at 37℃. Components of the pre-confluent medium included 1) 10% FBS/DMEM containing 4.5 g glucose/L; 2) 50 U/mL penicillin; and 3) 50 µg/mL streptomycin. Following confluence, a differentiation medium consisting of 10% FBS/DMEM containing 4.5 g glucose/L, 0.5 mM methylisobutylxanthine, 0.5 µM dexamethasone and 10 µg/mL insulin was added and the cells were forced to differentiate into adipocytes. Three days later, the medium was changed to a post-differentiation medium (10 µg/mL insulin in 10% FBS/DMEM).
A dose-dependent, response surface model (RSM) was used to assess the optimal antiinflammatory combinations of RIAA and PAC. Post-differentiation, day 6 adipocytes were treated with test material and in 49 RIAA:PAC combinations 4 h prior to the addition of TNFα at a final concentration of 10 ng/mL. A 7-dose matrix was constructed with RIAA at 20, 10, 5, 2.5, 1.25, 0.625 or 0.313 µg/mL and PAC at 50, 25, 12.5, 6.25, 3.13, 1.58 or 0.79 µg/mL. Following incubation for approximately 20 h, the supernatant media were assayed for glycerol and IL-6 (vide infra). Results were adjusted for viable cells as determined by crystal violet staining.
The RSM was constructed for the observed response relative (ORR) to the TNFα control minus the expected response relative (ERR) to the TNFα control as determined from the additive composite curve. ERR values were derived from an internal, single compound curve for both RIAA (compound A) and PAC (compound B) and were performed in the same trials as the mixture results. For the construction of the additive composite curve of compounds A and B, the Loewe additive estimate of the expected effects of the various combinations was made using the relationship: [1/EAB] = [pA/EA] + [pB/EB], where EA and EB represented the observed effects, respectively, for A and B, while pA and pB represented the relative fractions of each component in the test mixture and pA + pB = 1 [19]. Thus, the plane representing ORR - ERR = 0 describes a response surface in which the two components were producing the expected, additive response. Values above the plane describe a greater than expected stimulatory effect of the combination of components and values below the plane describe an inhibitory effect of the components that was greater than expected [20].
TNFα-induced FFA release from 3T3-L1 adipocytes was quantified by measuring glycerol secretion into the medium. Glycerol was measured spectrophotometrically using the Free Glycerol Determination Kit (F6428, Sigma) and an EL 312e Microplate BIOKINETICS spectrophotometer (BioTek, Winooski, VT). Control, non-stimulated D6/D7 3T3-L1 adipocytes produced, on average, 772 ng glycerol/mL over 20 h. Treatment with 10 ng TNFα/mL historically induced a 50 to 300% increase in glycerol secretion relative to controls in this laboratory with this protocol.
After the optimal ratio of RIAA and PAC was determined, a second experiment was conducted to compare the glycerol secretion after cells were pre-incubated with RIAA (5 µg/mL), PAC (6.25 µg/mL), RIAA:PAC 5:1 (6.25 µg/mL) or troglitazone (5 µg/mL), using the same glycerol assay described above.
The IL-6 secreted into the medium in response to TNFα stimulation was quantified using the Quantikine® Mouse IL-6 Immunoassay kit with no modifications (R&D Systems, Minneapolis, MN). Information supplied by the manufacturer indicated that recovery of IL-6 spiked in mouse cell culture media averaged 99% with a 1:2 dilution and the minimum detectable IL-6 concentration ranged from 1.3 to 1.8 pg/mL. All supernatant media samples were diluted 1:30 for quantification.
The nuclear stain crystal violet was used to assess cytotoxicity and adjust for cell viability [21]. Wells of the microtiter plates were washed twice with PBS and air-dried at ambient temperature for 5 min. One-hundred µL of a 0.3% crystal violet solution in 20% methanol were added to each well and incubated for an additional 30 min at ambient temperature, wells were then washed 1 time with PBS for 5 min and treated with 100 µL of a 1% sodium dodecyl sulfate solution on a shaker for 1 h. Absorbance was read on a spectrophotometer at 595 nm.
Mature 3T3-L1 adipocytes maintained in post-differentiation medium consisting of 10% FBS/DMEM were placed in 0.5% BSA for 24 h. Five hours prior to the addition of 10 ng TNFα/mL, cells were treated with 5 µg of a 5:1 combination of RIAA: PAC/mL, 5 µg pioglitazone/mL (for IR and IRS-1) or 5 µg rosiglitazone/mL (for PI3K). Thiazolidinediones were used as the positive controls; as such each assay was optimized for the thiazolidinedione that would produce the greatest effect size to maximize the dynamic range of the assay. For the IR and IRS-1 phosphorylation assays, after cells were treated with test materials overnight (18-24 h), 100 nM insulin was added for 15 minutes and lysates were prepared. Phosphorylation of IR tyrosines 1162 and 1163 and IRS-1 serine 307 was quantified using the PhosphoDetect™ Insulin Receptor (pTyr1162/1163) ELISA Kit (Calbiochem) and the BioSource IRS-1 [pS307] ELISA (Camarillo, CA), respectively. For the PI3K assay, after cells were treated with test materials overnight (18-24 h), 100 nM insulin was added. Cells were then fixed after 15 min. Phosphorylated and total PI3K were quantified using the respective FACE™ ELISA kit (Active Motif, Carlsbad, CA) as instructed by the manufacturer. Both phosphorylated and total proteins were quantified and phosphoprotein content was normalized to total protein. Analysis was performed using the log-normal transformation of the mOD450 values per well or corrected for viable cells using crystal violet.
Glucose uptake in insulin-resistant 3T3-L1 adipocytes was measured using the fluorescent D-glucose derivative, 2-NBDG as described by Leira et al. [22]. D17 3T3-L1 adipocytes were serum-starved in 0.5% BSA for 24 h and pre-treated with 5 µg of a 5:1 combination of RIAA:PAC/mL, 5 µg pioglitazone/mL (positive control) or 901 µg aspirin/mL (positive control) for 18 h in 96-well microtiter plates. Insulin stimulation consisted of a 5-minute incubation with 100 nmol insulin/L following which cells were treated with 100 µmol/L 2-NBDG for 5 min and immediately washed 3 times with PBS. A Packard Fluorocount spectrofluorometer (Model#BF10000, Meridan, CT) with a 485 nm excitation filter and 530 nm emission filter was used for quantification of 2-NBDG fluorescence. Experiments were performed a minimum of 6 times. For comparison across experiments with different positive controls, relative fluorescent units were normalized to the PBS/solvent control.
The effect of RIAA and PAC on nonfasting serum glucose and insulin was assessed in the db/db mouse model performed at MDS Pharma Services (Taiwan, ROC). Groups of five, 9-week-old, male mice (C57BLKS/J-m+/+ Leprdb), weighing 50 ± 5 g, were provided by the Institute for Animal Reproduction (IAR, Japan). The animals were housed in Individually Ventilated Cages Racks (IVC Racks, 36 Mini Isolator systems) throughout the experiment. Each APEC® cage (in cm, 26.7 length × 20.7 width × 14.0 height) was sterilized in an autoclave and housed five mice. The mice were maintained in a hygienic environment under controlled temperature (22 - 24℃) and humidity (60% - 70%) with 12-h light/dark cycle. The animals were fed sterilized lab chow and sterilized distilled water ad libitum. All aspects of this work, including housing, experimentation and disposal of animals, were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington, D. C., 1996).
Animal body weight was measured at the beginning and at the end of the feeding period. RIAA, PAC, 5 selected combined RIAA/PAC (ranging from 1:5 to 5:1; at 100 mg/kg), vehicle (2% Tween 80, Wako, Japan), metformin (positive control; at 100 mg/kg and 300 mg/kg), and rosiglitazone (positive control; at 0.5 mg/kg) were administered orally daily by gavage for 7 consecutive days starting immediately after the pre-treatment blood sampling (day 1). Post-treatment blood samples were drawn from the orbital sinus 90 min after administration of the final dose on day 7. Serum glucose and insulin levels were determined by enzymatic (Mutaratase-GOD, Wako, Japan) and ELISA (SPI-bio, France) methods, respectively.
In vitro data were analyzed using a one-way ANOVA; Fisher's least significant difference test was used to determine differences from the respective control. The probability of a type I error was set at the nominal 5% level. For in vivo data, the statistical significance of reductions in nonfasting serum glucose and insulin as well as Homeostatic Model Assessment of Insulin Resistance (HOMA) scores was assessed by computing the least significant difference (lsd) (Excel, Microsoft, Redmond, WA) for the percent decrease from pre-test values of the two independent, concurrent control groups. With a probability of a type I error set at the nominal 5% level, the lsd for percent decrease in serum glucose and insulin were 1.16 and 7.17%, respectively.
Over the 49 combinations tested, the general shapes of the RSM (Fig. 1A, B) revealed a complex interaction between RIAA and PAC resulting in both anti- and pro-inflammatory effects in TNFα-stimulated 3T3-L1 adipocytes. For both inhibition of lipolysis (Fig. 1A) and inhibition of IL-6 secretion (Fig. 1B), there existed an asymmetry in the response surface where the inhibitory effects of RIAA were increased at low concentrations of PAC and eliminated with increasing PAC concentrations. Although both RSMs were distinct for TNFα-stimulated lipolysis and IL-6 secretion, the regions of enhanced inhibition occurred at similar ratios of RIAA:PAC. For the inhibition of lipolysis, there existed a trough at 5 µg RIAA/mL through PAC concentrations of 0.78 to 12.5 µg/mL, or ratios of RIAA:PAC of 6.4:1 to 0.4:1. Optimal inhibition of IL-6 secretion occurred at 1.58 µg PAC through all concentrations of RIAA, or ratios of RIAA:PAC of 6.3:1 to 0.4:1. The region of overlap between the two RSMs existed between ratios of 6.4:1 to 3.2:1. Based upon these results, further studies utilized a 5:1 combination of RIAA:PAC.
Treatment of D6 3T3-L1 adipocytes with 10 ng TNFα/mL overnight induced lipolysis resulting in the release of FFA into the supernatant media. Pretreatment of 3T3L1 adipocytes with troglitazone, RIAA, PAC, and the 5:1 RIAA:PAC mixture 1 h prior to TNFα stimulation reduced the extent of lipolysis by 43% (P < 0.01), 22%, (P < 0.01), 11% (P < 0.05), and 45% (P < 0.01), respectively (Fig. 2). The anti-lipolytic effect of the mixture was similar to that of troglitazone and greater than the sum of percent activities of the extracts alone.
Insulin and insulin + TNFα exposure led to relative increases in IR tyrosine 1162 or 1163 phosphorylation (Fig. 3A). Pretreatment with 5:1 RIAA:PAC mixture had no effect on IR phosphorylation induced by either insulin alone or insulin + TNFα. In contrast, pioglitazone increased IR phosphorylation by 63% (P < 0.01) in unstimulated adipocytes and 22% (P < 0.01) in cells stimulated by insulin alone.
In unstimulated adipocytes, the 5:1 RIAA:PAC mixture decreased IRS-1 phosphorylation by 41% (P < 0.01). Phosphorylation of IRS-1 serine 307 was increased by exposure to both insulin and insulin + TNFα. The mixture attenuated this increase by 34% (P < 0.01) and 17% (P < 0.01), respectively (Fig. 3B). Pioglitazone produced a similar degree of inhibition in unstimulated cells (55%, P < 0.01) and in the presence of insulin alone (31%, P < 0.01), and a stronger inhibition (38%, P < 0.01) in the presence of insulin + TNFα than the mixture.
Neither insulin nor insulin + TNFα had any effect on PI3K phosphorylation (Fig. 3C). In presence of insulin, 5:1 RIAA:PAC increased PI3K phosphorylation by 33% (P < 0.01), compared with 41% (P < 0.01) for rosiglitazone; this effect was reduced in cells exposed to TNFα for both the mixture and rosiglitazone, but still increased similarly relative to controls (P < 0.05).
Treatment of 3T3-L1 adipocytes with the mixture increased insulin-stimulated glucose uptake 3.0-fold (P < 0.05) relative to untreated adipocytes. This increase was quantitatively similar to those observed with 5.0 µg pioglitazone/mL (2.8-fold, P < 0.05) or 901 µg aspirin/mL (3.8-fold, P < 0.05) (Fig. 4).
Animal body weight in each feeding group did not change before and after the 7-day feeding period and there were no differences between each feeding group (data not shown). Metformin (100 mg/kg and 300 mg/kg) and rosiglitazone (0.5 mg/kg) decreased both nonfasting serum glucose and insulin concentrations, as well as HOMA scores, relative to the solvent controls over 7 d in the db/db mouse model (Fig. 5). The decreases in serum glucose, insulin and HOMA scores produced by metformin were dose-related. At 0.5 mg rosiglitazone/kg decreased serum glucose by 28% (P < 0.01), insulin by 9.5% (P < 0.05), and HOMA scores by 37% (P < 0.01). Individually, RIAA and PAC at 100 mg/kg for 7 d reduced serum glucose 7.4 and 7.6% relative to controls, respectively (P < 0.05). The 1:5, 1:2 and 1:1 ratios of RIAA:PAC produced decreases in glucose (P < 0.05); however, their effects were less than those of either RIAA or PAC alone. In contrast, the 2:1 and 5:1 ratios of RIAA:PAC decreased serum glucose by 11 and 22%, respectively, relative to controls (P < 0.01).
The 2:1 and 5:1 RIAA:PAC combinations reduced serum insulin by 17 and 19% relative to the controls, respectively (P < 0.01). These decreases were comparable to that observed with 300 mg metformin/kg. The 1:5 RIAA:PAC combination was associated with a 9.3% reduction relative to controls (P < 0.05), which was comparable to the effect of rosiglitazone, and slightly lower than that of either PAC alone (11%, P < 0.05) or 100 mg metformin/kg. RIAA alone showed no effect on serum insulin.
Individually, RIAA and PAC did not significantly affect HOMA scores. However, at 5:1 ratio, RIAA:PAC reduced HOMA scores by 38% relative to controls (P < 0.01), comparable to the effect of rosiglitazone.
The American Heart Association recommends lifestyle therapies as first-line interventions to manage metabolic syndrome. For example, both Mediterranean diet and physical activity have been shown to improve insulin sensitivity and provide other cardiometabolic benefits [23,24]. When lifestyle modification alone is insufficient or ineffective, pharmacologic agents are recommended. Long-term pharmacotherapy frequently involves multiple drugs. Unfortunately, such chronic polypharmacy may lead to increased risk of adverse drug reactions and interactions [25]. Further, drugs treating insulin resistance and diabetes tend to lose their effectiveness on glucose control over time [26]. Therefore, there continues to be a need for alternative treatments. Another logical approach is combination therapies; finding synergy between two (or more) compounds so that lower doses of each are administered to maximize therapeutic effect while reducing the potential for adverse effects associated with either compound alone. This approach is frequently used in antimicrobial and anticancer combination therapy.
In a previous study following this principle, RIAA and PAC were combined and tested in animal models and in a human clinical trial [27]. However, the ratio of RIAA to PAC used in that study was based on earlier clinical experiences from individual case studies. In addition, the animals only received RIAA and PAC for 3 days. In this study, we evaluated 49 concentration combinations of RIAA and PAC in a 7-dose matrix and constructed a RSM to identify regions of interactions. This method allowed for a more objective assessment of the relative potencies of the two compounds in vitro and helped to empirically identify optimal combination(s) for further scientific evaluations, including an in vivo evaluation in which mice were fed RIAA and PAC for 7 days. Here we report that their combination at a 5:1 ratio exhibited greatest potency for inhibiting FFA and IL-6 secretion in TNFα-stimulated 3T3-L1 adipocytes.
Central (visceral) obesity is strongly correlated with insulin resistance; the increased release of FFA into the portal vein and liver from the visceral fat has a detrimental effect on insulin action [28]. Several studies have supported this 'portal theory' [29-31]. It has been demonstrated that compounds that inhibit FFA release from adipose tissue improve insulin sensitivity and ameliorate at least some of the symptoms of type 2 diabetes [32]. Hence, in our first series of experiments (Fig. 2), we employed TNFα-stimulated, mature 3T3-L1 adipocytes to compare the effect of RIAA, PAC, and their 5:1 combination on secretion of FFA. This model incorporates the two common features of type 2 diabetes; clinically elevated levels of TNFα and adipose tissue dysfunction. RIAA and PAC individually inhibited FFA release, and the 5:1 combination resulted in a reduction greater than the sum of its components, consistent with the RSM.
Next, we investigated whether the RIAA:PAC 5:1 mixture modulated the insulin signaling cascade in 3T3-L1 adipocytes (Fig. 3). Normally, when insulin is present, IR stimulates tyrosine phosphorylation of IRS proteins, leading to activation of downstream signaling pathways such as the PI3K/Akt pathway [10]. In insulin-resistant states, serine 307 phosphorylation of the IRS proteins is increased, causing inhibition and termination of downstream insulin signaling and resulting in the disruption of normal metabolic functions [33]. The presence of the inflammatory cytokine TNFα further stimulates serine 307 phosphorylation of the IRS proteins [34,35]. In this experimental model, we observed both similarities and differences between RIAA:PAC and thiazolidinedione drugs in effects in insulin signaling. Insulin induced IR tyrosine phosphorylation, and pretreatment with the insulin-sensitizing agent pioglitazone enhanced the IR tyrosine phosphorylation as expected (Fig. 3A). The RIAA/PAC combination, on the other hand, did not affect IR phosphorylation. Both RIAA/PAC and pioglitazone significantly suppressed the induction of IRS-1 serine 307 phosphorylation in all testing conditions (Fig. 3B); RIAA/PAC appeared to be slightly less potent than pioglitazone in the presence of TNFα. Both RIAA/PAC and rosiglitazone increased PI3K phosphorylation in the presence of insulin and insulin plus TNFα (Fig. 3C); rosiglitazone appeared to be more potent in the presence of TNFα. Phosphorylated PI3K leads to the production of phosphatidylinositol-3,4,5-triphosphate, which is responsible for the activation of Akt signaling [36]. The activated Akt phosphorylates several substrates (such as GSK3) and hence controlling various functions including glycogen synthesis, glucose uptake, and protein synthesis [10].
While we made some observations on their effects in insulin signaling in this study, several limitations and questions should be addressed in order to elucidate the biological mechanisms of RIAA and PAC. First, Western blot method would be more appropriate to confirm the observed changes of insulin signaling. Second, there is a need to understand how RIAA/PAC affects IRS via IR-independent pathway(s). Third, how RIAA/PAC affects downstream Akt-mediated signaling should be investigated. These will help us understand why 5:1 ratio, as opposed to other ratios, exhibited the strongest biological effects.
Because in vitro findings are not always applicable to in vivo settings due to potential differences in bioavailability and metabolism in RIAA and PAC, we tested the mixture (in addition to the individual RIAA and PAC) in 5 combinations in vivo, including the 5:1 ratio. In db/db mice fed with 100 mg/kg RIAA for 7 d, we observed a significant decrease in serum glucose and a trend toward decrease in insulin (but did not reach statistical significance). Mice fed with 100 mg/kg PAC exhibited significant reductions in both serum glucose and insulin. Among the 5 RIAA:PAC combinations tested in these animals, the most potent effect was observed at the 5:1 ratio, which is in line with our in vitro results. The RIAA and PAC 5:1 combination exhibited efficacy similar to that of 300 mg/kg of metformin or 0.5 mg/kg of rosiglitazone. This suggests that, working together, RIAA and PAC may have therapeutic potential.
RIAA and PAC are derived from plants that have a long history of safe dietary use in humans. Both achieved self-affirmed Generally Recognized As Safe (GRAS) status, and RIAA has been approved by the FDA as beer flavoring agent under Section 21 CFR 172.560. Further, in a 12-week randomized control trial, RIAA and PAC (at a 5:1 ratio; 300 mg RIAA and 60 mg PAC per day) in addition to a medical food as part of a therapeutic lifestyle change program resulted in significantly greater improvements in lipid markers associated with metabolic syndrome and cardiovascular disease as compared to the therapeutic lifestyle change program alone, and no adverse events were reported [18].
In our in vitro study, we only assessed FFA release and glucose uptake in 3T3-L1 adipocytes. Given the interconnection among liver, muscle and adipose tissues in the development of insulin resistance, additional research using mouse/rat adipocytes, liver and muscle tissues would provide valuable information on the mechanism(s) of action of RIAA/PAC. Additionally, in the animal experiment we only measured changes in serum glucose and insulin levels but not glucose tolerance test, changes in circulating free fatty acids, or other inflammatory markers (e.g. IL-6 and TNFα). Those measurements would provide additional relevant links to the proposed biological mechanism(s). Moreover, the dose used in our animal study (100 mg/kg) was relatively high. Follow-up studies in humans should address pharmacokinetics, optimal dose for therapeutic benefit, and long-term safety and efficacy of the compounds.
Our study shows that the RIAA:PAC mixture at 5:1 ratio (w/w) increases the inhibition of TNFα-stimulated lipolysis and enhances glucose uptake in 3T3-L1 adipocytes, modulates insulin signaling pathways in 3T3-L1 adipocytes via reducing the elevated IRS-1 serine phosphorylation and increasing PI3K phosphorylation, and reduces serum glucose and insulin concentrations in db/db mice. RIAA/PAC mixture may have the potential to be an effective alternative when conventional therapy is undesirable or ineffective. Future research will explore the long-term clinical application of RIAA/PAC mixture as a novel nutritional approach to help manage metabolic syndrome, insulin resistance, and type 2 diabetes.
Figures and Tables
Fig. 1
Dose-dependent, response surface representation of the interaction between RIAA and PAC on (A) free-fatty acids secretion as determined by glycerol release, and (B) IL-6 secretion. Detailed description of the response surface models (RSM) please refer to Methods. Values above the plane described a greater than expected stimulatory effect of the combination of components and values below the plane describe an inhibitory effect of the components greater than expected.

Fig. 2
RIAA, PAC and the 5:1 mixture of RIAA:PAC inhibit TNFα-stimulated FFA release in 3T3-L1 adipocytes. Data were analyzed using a one-way ANOVA; Fisher's least significant difference test was used to determine differences from the respective control. Data are presented as means ± 95% CI. *P < 0.05, **P < 0.01.

Fig. 3
RIAA:PAC at 5:1 effects on (A) IR, (B) IRS-1, and (C) PI3K in mature 3T3-L1 adipocytes. Both phosphorylated and total proteins were quantified and phosphoprotein content was normalized to total protein. Analysis was performed using the log-normal transformation of the mOD450 values per well or corrected for viable cells using crystal violet. Values presented represent the means ± 95% CIs. *P < 0.05, **P < 0.01 compared to DMSO.
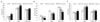
Fig. 4
The 5:1 combination of RIAA:PAC on insulin-stimulated glucose uptake in 3T3-L1 adipocytes. For comparison across experiments with different positive controls, RFU were normalized to the PBS/solvent control and viable cells within each experiment. Data are presented as means ± 95% CI using pioglitazone and aspirin as the positive controls. *P < 0.05 compared to insulin alone.

References
1. Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005. 28:2745–2749.

3. Isomaa B, Henricsson M, Almgren P, Tuomi T, Taskinen MR, Groop L. The metabolic syndrome influences the risk of chronic complications in patients with type II diabetes. Diabetologia. 2001. 44:1148–1154.

4. DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991. 14:173–194.

5. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003. 112:1821–1830.

6. Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008. 28:1654–1659.

7. Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007. 56:1010–1013.

8. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995. 95:2409–2415.

9. McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002. 51:7–18.
10. Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006. 7:85–96.

12. Bailey CJ. Treating insulin resistance in type 2 diabetes with metformin and thiazolidinediones. Diabetes Obes Metab. 2005. 7:675–691.

13. Doggrell SA. Clinical trials with thiazolidinediones in subjects with Type 2 diabetes--is pioglitazone any different from rosiglitazone? Expert Opin Pharmacother. 2008. 9:405–420.

14. Amiel SA, Dixon T, Mann R, Jameson K. Hypoglycaemia in Type 2 diabetes. Diabet Med. 2008. 25:245–254.

15. Hernandez AV, Usmani A, Rajamanickam A, Moheet A. Thiazolidinediones and risk of heart failure in patients with or at high risk of type 2 diabetes mellitus: a meta-analysis and meta-regression analysis of placebo-controlled randomized clinical trials. Am J Cardiovasc Drugs. 2011. 11:115–128.

16. Babish JG, Pacioretty LM, Bland JS, Minich DM, Hu J, Tripp ML. Antidiabetic screening of commercial botanical products in 3T3-L1 adipocytes and db/db mice. J Med Food. 2010. 13:535–547.

17. Konda VR, Desai A, Darland G, Bland JS, Tripp ML. Rho iso-alpha acids from hops inhibit the GSK-3/NF-kappaB pathway and reduce inflammatory markers associated with bone and cartilage degradation. J Inflamm (Lond). 2009. 6:26.

18. Lerman RH, Minich DM, Darland G, Lamb JJ, Schiltz B, Babish JG, Bland JS, Tripp ML. Enhancement of a modified Mediterranean-style, low glycemic load diet with specific phytochemicals improves cardiometabolic risk factors in subjects with metabolic syndrome and hypercholesterolemia in a randomized trial. Nutr Metab (Lond). 2008. 5:29.

19. Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953. 3:285–290.
21. Flick DA, Gifford GE. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984. 68:167–175.

22. Leira F, Louzao MC, Vieites JM, Botana LM, Vieytes MR. Fluorescent microplate cell assay to measure uptake and metabolism of glucose in normal human lung fibroblasts. Toxicol In Vitro. 2002. 16:267–273.

23. Burr JF, Rowan CP, Jamnik VK, Riddell MC. The role of physical activity in type 2 diabetes prevention: physiological and practical perspectives. Phys Sportsmed. 2010. 38:72–82.

24. Esposito K, Giugliano D. Mediterranean diet and the metabolic syndrome: the end of the beginning. Metab Syndr Relat Disord. 2010. 8:197–200.

26. Turner RC, Cull CA, Frighi V, Holman RR. UK Prospective Diabetes Study (UKPDS) Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999. 281:2005–2012.

27. Minich DM, Lerman RH, Darland G, Babish JG, Pacioretty LM, Bland JS, Tripp ML. Hop and Acacia Phytochemicals Decreased Lipotoxicity in 3T3-L1 Adipocytes, db/db Mice, and Individuals with Metabolic Syndrome. J Nutr Metab. 2010. 2010:pii: 467316.
29. Bergman RN, Kim SP, Hsu IR, Catalano KJ, Chiu JD, Kabir M, Richey JM, Ader M. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med. 2007. 120:S3–S8. discussion S29-32.

30. Kabir M, Catalano KJ, Ananthnarayan S, Kim SP, Van Citters GW, Dea MK, Bergman RN. Molecular evidence supporting the portal theory: a causative link between visceral adiposity and hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2005. 288:E454–E461.

31. Rytka JM, Wueest S, Schoenle EJ, Konrad D. The portal theory supported by venous drainage-selective fat transplantation. Diabetes. 2011. 60:56–63.

32. Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, DeFronzo RA. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes. 2005. 54:3148–3153.

34. Hotamisligil GS, Budavari A, Murray D, Spiegelman BM. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J Clin Invest. 1994. 94:1543–1549.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download