Abstract
High consumption of fruits and vegetables has been suggested to provide some protection to smokers who are exposed to an increased risk of numerous cancers and other degenerative diseases. Carrot is the most important source of dietary β-carotene. Therefore, the objective of this study was to investigate whether carrot juice supplementation to smokers can protect against lymphocyte DNA damage and to compare the effect of supplementation of capsules containing purified β-carotene or a placebo (simple lactose). The study was conducted in a randomized and placebo-controlled design. After a depletion period of 14 days, 48 smokers were supplemented with either carrot juice (n = 18), purified β-carotene (n = 16) or placebo (n = 14). Each group was supplemented for 8 weeks with approximately 20.49 mg of β-carotene/day and 1.2 mg of vitamin C/day, as carrot juice (300 ml/day) or purified β-carotene (20.49 mg of β-carotene, 1 capsule/day). Lymphocyte DNA damage was determined using the COMET assay under alkaline conditions and damage was quantified by measuring tail moment (TM), tail length (TL), and% DNA in the tail. Lymphocyte DNA damage was significantly decreased in the carrot juice group in all three measurements. The group that received purified β-carotene also showed a significant decrease in lymphocyte DNA damage in all three measurements. However, no significant changes in DNA damage was observed for the placebo group except TM (P = 0.016). Erythrocyte antioxidant enzyme was not significantly changed after supplementation. Similarly plasma lipid profiles were not different after carrot juice, β-carotene and placebo supplementation. These results suggest that while the placebo group failed to show any protective effect, carrot juice containing beta-carotene or purified β-carotene itself had great antioxidative potential in preventing damage to lymphocyte DNA in smokers.
Recently, studies on the effect of antioxidant foods and health functional foods on the prevention and treatment of various chronic diseases have been conducted. Such substances have been reported to possess antioxidant function and normalize compromised antioxidant systems by the oxidative stress caused by free radicals. Particularly, smoking causes an abnormal increase of free radical, which causes strong oxidation in the body and damages lipids, proteins, or even cells. Therefore, the antioxidant nutrition status is crucial for smokers, and it is critical to consume enough antioxidant nutrients to continuously neutralize free radicals.
When oxidizing damage continuously occurs, it causes DNA damage in cells, which raises the risk of diseases such as aging or cancer [1,2]. Therefore, research on the effects of supplementation of antioxidant food on a smoker's antioxidant nutrition status need to be conducted. Recently, Riso et al. [3] reported that the the oxidized purines in smokers were reduced significantly to the level of non-smokers, when supplementing broccoli. Wang et al. [4] also reported that the serum radical level decreased significantly when smokers consumed noni juice for 30 days. Also, consumption of 250 g of broccoli by young smokers over 10 days was shown to protect against DNA damage [5]. In contrast, a previous study [6] reported that supplementation of mixed vegetable and fruit had no effect on decreasing DNA damage.
While studies have been conducted to improve the smoker's antioxidant status by directly using food supplements, some studies have evaluated the effects of refined vitamins, which have a higher bioavailability than food material. Jacobson et al. [7] reported that the level of DNA damage was reduced significantly after smokers consumed refined vitamins (Vitamin C, E, β-carotene), and Lee et al. [8] also observed a reduction in the level of DNA after heavy smokers consumed vitamins for 6 months. In another intervention research [9], it was reported that the level of DNA damage was reduced significantly after taking a vitamin C supplement for a certain period of time. However, in the ATBC (Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study) [10] long-term supplementation of β-carotene was reported to have no effect in reducing lung cancer in smokers, and may have even increased the risk of lung cancer. Also, supplementation of high amounts of vitamin E increased the risk of tuberculosis in heavy smokers. Thus, previous research has demonstrated the effect of nutrition supplementation on smokers using vegetables such as tomato, carrot, spinach, etc. rather than supplying refined antioxidant nutrients such as vitamin C, E, β-carotene, etc., however, the most effective method still remains controversial.
Thus, this study was conducted to compare the effects of antioxidant vitamin and vegetable supplementation, by measuring the lymphocyte DNA damage level and antioxidant nutrition status before and after supplementation with vegetables containing β-carotene or the same amount of vitamin in smokers with high DNA damage over 8 weeks.
This research has been carried out over 8 weeks using male staff smokers from the H University and the Korea Water Resources Corporation located in the Daejeon area. The subject's ages were between 23-57. Among the 50 subjects who had responded to the survey in the beginning, 48 smokers were used for the study, where 2 subjects who decided not to participate due to an unexpected long-term business trip were excluded. The inclusion criteria for the subjects were subjects without hereditary or chronic diseases and without ill symptoms or diagnosis. The survey was processed after receiving the subjects' own written consent. The contents of questionnaire included general questions about the subjects' age and health state, exercising habit, smoking and alcohol drinking status, supplementation of vitamin or nutrient, etc. Smokers who had been smoking 8 cigarettes or more a day for 3 years at least were selected as subjects, and the amount of drinking was calculated using the unified international unit of drink/day, which was also based on the type of alcohol consumed. 1 drink was based on about 14% of 100% alcohol, which corresponds to 1/2 ounce of 100% alcohol. This means that 1 cup (55 cc) of soju, 2 hops and 1 bottle (350 cc) of beer, or 5 hop of makgeolli (rice fermented alcohol) was considered 1 drink. Exercising time/day was calculated by multiplying routine exercising time per exercise by the exercising frequency through the survey. Height, weight was measured and BMI (body mass index, kg/m2) was calculated. WHR (waist hip ratio), body-fat percentage (%), and blood pressure were measured.
Dietary assessment was conducted by 1:1 interview using the 24 hour recall method before (0 week) and after (8 weeks) supplementation of carrot juice and β-carotene. The interview was carried out by the department of food & nutrition. To assist in recalling the amount of food consumed, food model and the approximate amount of food viewed on a photo were presented during the survey for all food types. Total nutrient intake was estimated using the CAN program 2.0 (Nutrition Information Center, Korean Nutrition Society).
Food frequency questionnaire was used to survey flavonoid and carotenoid contents consumed by the subjects over 1 month. The database of flavonoid and carotenoid contents in the food was referred to the isoflavone content in the food [11] as published by the USDA-Iowa State University/USA, isoflavones data base [12] of Korea by Kim and Yoon, and the flavonoid contents per food reported in the preceding researches by Hertog et al. [13-15] and Bilyk et al. [16,17]. A questionnaire for semi-quantitative food supplementation frequency survey [18] developed by this laboratory in 2001 was distributed to the 50 smokers. Average flavonoid and carotenoid amount taken in each day by the subjects was calculated using the flavonoid content database of each food after calculating the daily average food amount per individual by multiplying the 1 time feeding amount and feeding frequency value for each survey subject.
This study was conducted over 8 weeks after dividing the 50 smoking subjects into carrot juice group, β-carotene group, and placebo group. Two bottles (total 300 ml) of commercial 100% carrot juice were supplemented every day to the carrot juice group. For the β-carotene group, tablets containing β-carotene (20.49 mg) and vitamin C (1.2 mg) were provided, which is the same amount in 300 ml of the carrot juice (Table 1). The tablets made up of 150 mg lactose in the same form as the vitamin tablet were supplemented to the placebo group. The contents of β-carotene and vitamin C in the carrot juice used in this research were analyzed by the method described by Kim et al. [19]. Using this analysis, the juice was shown to contain 6.83 mg/100 g of β-carotene and 0.4 mg/100 g of vitamin C. Fresh carrot juice was delivered to each survey subject every morning and they were told to drink 2 bottles during the day. The β-carotene and placebo were provided a 1 pill every day. The subjects were instructed to consume the pill every day and to record a daily log while consuming the carrot juice, antioxidant vitamin, or placebo. A depletion period was set up for 2 weeks, where consumption of fruits and vegetables was restricted before supplementation with carrot juice, β-carotene, or placebo. This was done so the subjects had a similar antioxidant vitamin status. Also, they were reminded and advised over the phone individually to refrain from supplementing meals or foods during the experimental period, which may affect the antioxidant index.
Blood was drawn for the subjects before (0 week) and 8 weeks after the supplementation of carrot juice, β-carotene, and placebo. Whole blood samples that were drawn from the survey subjects after fasting were placed in a 10 ml heparinated sterile tube (Becton Dickinson Co.), and brought to the laboratory. Some of the whole blood was placed separately for Comet analysis to measure DNA damage. The remaining blood was centrifuged at 1,000 rpm for 15 minutes to collect the PRP (platelet-rich plasma) on the top for vitamin C analysis, it was then centrifuged again at 3,000 rpm for 30 minutes to collect the PDP (platelet-deficient plasma) for the separation of blood plasma. The blood plasma was divided for each analysis item and kept at -80℃ in the freezer until used. Erythrocytes were centrifuged 3 times with iso-osmotic phosphate buffered saline (pH 7.4) for 10 minutes at 3,000 rpm. The erythrocyte suspension and plasma was divided for each analysis and kept at -80℃ in a freezer until used.
Lymphocytes were separated using 100 µl of Histopaque 1077, after mixing 70 µl of the fresh whole blood in 900 µl of PBS. The isolated lymphocytes duplicated from 1 subject were sujected to oxidative stress by suspension in phosphate-buffered saline with 100 µmol/l H2O2 for 5 min on ice. The lymphocytes were mixed with 75 µl of 0.7% low-melting agarose and added to the slides precoated with 0.5% agarose. The slides were then immersed in a lysis solution (2.5 mol/l NaCl, 100 mmol/l EDTA, 10 mmol/l Tris, 1% sodium laurylsarcosine, 1% Triton X-100, and 10% dimethyl sulfoxide) for 1 h at 4℃. The slides were placed into an electrophoresis tank containing 300 mmol/l NaOH and 10 mmol/l Na2-EDTA (pH 13.0) for 40 min. For electrophoresis of the DNA, an electric current of 25V/300 ± 3 mA was applied for 20 min at 4℃. The slides were washed three times with a neutralizing buffer (0.4 mol/l Tris, pH 7.5) for 5 min at 4℃ and then treated with ethanol for another 5 min before staining with 50 µl ethidium bromide (20 µg/ml). The slides were then viewed under a fluorescent microscope (Leica, Germany). Images of the nucleus, which were acquired using a CCD camera (Nikon, Japan), were analyzed using a comet image analyzing system (Kinetic Imaging, UK). DNA damage in the lymphocyte and the damage restriction levels by supplementation of carrot juice and antioxidant vitamins were measures using 3 analysis indexes [20]: tail length (TL), which is the distance the DNA fragment moved from the nucleus, DNA in tail (% DNA), and tail movement (TM), which is the value obtained by multiplying TL and%DNA. The DNA damage degree was measured from a total of 100 lymphocytes (50 cells from each of two replicate slides).
The analysis of catalase, glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) in the red blood cell was carried out as follows. After treating the red blood cells with hydrogen peroxide, the amount of reduced hydrogen peroxide was measured at 240 nm for 30 seconds using a UV/VIS spectrophotometer [21]. GSH-Px catalyzes the oxidizing reaction of glutathione by the peroxide (t-butyhydroperoxide). In the downstream reaction, oxidized glutathione is restored to the glutathione in the presence of glutathione reductase and NADPH. Glutathione peroxidase was analyzed by measuring the reduced NADPH concentration after restoration at 340 nm for 90 seconds using a UV/VIS spectrophotometer [22]. For the SOD, ethanol and chloroform were added after the suspension of red blood cell was hemolyzed with distilled water, which was centrifuged at 3,000 U/min for 2 minutes. Pyrogallol was added to solutions of different concentrations and was measured at 320 nm for 180 seconds using a UV/VIS spectrophotometer [23].
Plasma lipid, total cholesterol and triglyceride contents were analyzed using the Photometric Auto analyzer (ERBA CHEM-PRO), from the plasma which had been kept in a -80℃ freezer. The samples were incubated with 1 ml of the enzyme solution provided in the kit (Somang Pharmaceutical Co., Ltd.) and reacted for 5 minutes in a constant temperature water bath. The HDL-Cholesterol was analyzed using the Photometric Auto analyzer (ERBA CHEM-PRO). In these experiments 0.2 ml of the blood plasma was mixed with 0.2 ml of precipitation reagent, incubated at room temperature for 5 minutes, centrifuging for 10 minutes, mixed with 0.1 ml of the top solution 0.1 ml and 3 ml of the enzyme solution, and reacted for 5 minutes in a constant temperature water bath at 37℃. LDL-Cholesterol was calculated using the Friedewald [24] equation.
All data was entered in an excel database system of MS, and the statistical tasks were performed using the SPSS-PC+ statistics package (version 17.0). Mean and standard error (SE) was obtained for each item, then the mean difference among the 3 groups was verified by Duncan after one-way ANOVA, and the statistical significance was evaluated at the level of α = 0.05. The significance of the mean comparison before and 8 weeks after each supplementation of carrot juice, antioxidant vitamin, and placebo was tested using a paired t-test. Also, chi-square tests were performed against the frequency of smoking and consumption of alcohol.
Table 2 shows the survey results of the general characteristics and lifestyle habits, including smoking and body measurements (height, weight, BMI, WHR, and body-fat percentage). The average age of the subjects was 36.5, which was not significantly different among each group. In addition, there was no significant difference in the height, weight, BMI, WHR, and obesity degree, which was measured based on body-fat percentage. The average smoking habit per day was 16.4 ± 1.2 in the carrot juice group, 19.0 ± 2.3 in the β-carotene group, and 16.5 ± 2.6 in the placebo group. In addition, the average smoking period (expressed as pack years) for the carrot juice group, β-carotene group and placebo group was 17.2 ± 2.4 years, 14.74 ± 1.9 years and 15.3 ± 2.6 years, respectively. The pack-year calculated based on 1 pack (20 cigarettes) for 1 year, when considering the smoking amount and smoking period, was 15.02 ± 2.1 for the carrot juice group, 16.18 ± 4.1 years for the β-carotene group, and 14.62 ± 4.35 years for the placebo group (Table 2). The percentage of subjects that consumed alcohol was 98%, and the alcohol intake was 35.8 ± 6.2 ml/day in the carrot juice group, 23.8 ± 5.3 ml/day in the β-carotene group, and 42.1 ± 13.5 ml/day in the placebo group (Table 2). In the survey of exercising habits, 56.25% (n = 27) exercised regularly 1~2 times a week and 43.75% (n = 21) rarely exercised. The exercising time 18.81 minutes/day for the carrot juice group, 18.21 minutes for the β-carotene group, and 19.90 minutes/day for the placebo group (Table 2). There was no significant differences before and 8 weeks after the experiment in the weight, BMI, WHR, etc. of all the groups. The diastolic and systolic blood pressures also did not show any significant differences after the intervention.
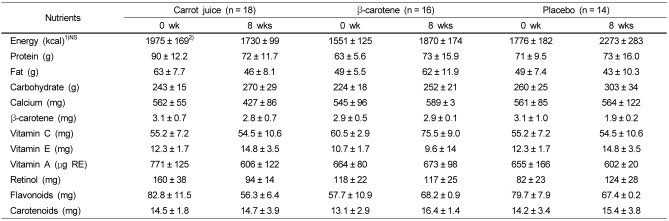
The dietary intake during the supplementation period was checked for any significant change among the three groups (Table 3). Before and 8 weeks after supplementation of carrot juice, β-carotene, and placebo, the subjects maintained their usual dietary consumption (Table 3). As a result, there was no change in the intake of flavonoid and carotenoid before and after supplementation of carrot juice and antioxidant vitamin (Table 3).
Lymphocyte DNA damage in the subjects before and after supplementation was measured using the Comet assay. DNA damage was assessed based on the tail length (TL), %DNA in the tail and tail moment (TM). There was no difference in the DNA damage level of the carrot juice, β-carotene, and placebo groups before supplementation. However, 8 weeks after the supplementation, the DNA damage level assessed by the TM (Fig. 1), DNA (Fig. 2) in tail (%) and TL (Fig. 3) of the carrot juice and β-carotene groups was significantly lower than the baseline. Reviewing the DNA damage recovery level before and 8 weeks after the supplementation, the TM value of carrot juice group was significantly reduced 8 weeks after supplementation (10.0 ± 0.4) when compared to the baseline at week 0 (17.7 ± 1.1). In addition, the DNA in tail (%) and TL were reduced significantly 8 weeks after supplementing with carrot juice (20.5 ± 0.5%, 52.3 ± 0.8 µM) when compared the baseline at week 0 (23.9 ± 0.7%, 71.0 ± 2.7 µM). In the β-carotene group, the TM was significantly reduced 8 weeks after vitamin supplementation (9.8 ± 0.6) when compared to the baseline at week 0 (18.8 ± 1.2) as was the DNA in tail (19.7 ± 0.6% vs 24.7 ± 0.8%). In addition, the TL decreased from 73.3 ± 2.5 µM to 53.3 ± 1.3 µM after supplementation for 8 weeks. In the case of the placebo group, the TM was reduced at 8 weeks after supplementation (14.4 ± 0.7) when compared to the baseline level at week 0 (17.9 ± 1.5), but no significant differences in the DNA in tail (%) was observed over this time period (23.3 ± 0.6% vs 24.5 ± 1.1%). Also there was no significant difference in TL before and after treatment (64.1 ± 1.8 µM vs 69.7 ± 3.3 µM).
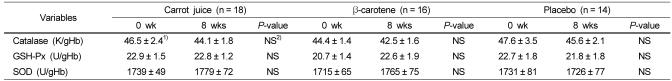
Erythrocyte catalase did not show any significant differences before and after supplementation with carrot juice, β-carotene, and placebo, and there was no difference in the values among the three groups (Table 4). The levels of GSH-Px and SOD were also not significantly different before and after supplementation for each group, and there was no difference among each group.
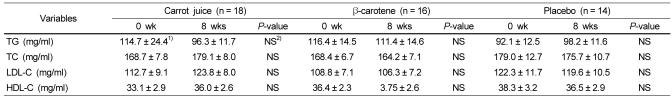
The plasma lipid levels for smokers after supplementation of carrot juice, β-carotene and placebo was analyzed. In this analysis, no significant changes in the levels of total cholesterol, TG, LDL-cholesterol, and HDL-cholesterol between all the groups was observed, and there was no difference among the groups (Table 5).
Due to the number of carcinogens and free radicals in the cigarette, oxidative stress is higher in smokers due to an increase of ROS in the body. This affects the antioxidant nutrition status in the body, which eventually results in DNA damage and an increase in the risk of chronic diseases. Numerous studies on supplementation of antioxidant substances and foods have been conducted with the goal of preventing the incidences of chronic diseases caused by smoking [25,26]. A study on DNA damage levels using the Comet assay between smokers and non-smokers was previously reported [27]. In addition, previous studies have also evaluated the degree of DNA damage in healthy vs. patients [28,29]. It has also been shown that antioxidant nutritional supplementation with watercress [30] and grape juice [31] or antioxidant pills such as vitamin E [32] were shown to mitigate DNA damage in smokers. While the refined antioxidant vitamin and antioxidant food products, like vegetables, have been shown to improve the antioxidant properties in the body of smokers, there has been no study comparing which supplementation is more effective. Therefore, this study was performed to compare the nutrition improving effects in the body after supplementation of vegetables and antioxidant vitamins in smokers.
In this study, when smokers consumed carrot juice, β-carotene, and placebo for 8 weeks and DNA damage levels were compared, both the carrot juice and β-carotene groups were shown to have improved DNA damage when compared with the placebo group. Supplementing carrot juice, antioxidant food, and refined β-carotene, as an antioxidant pill, have produced similar effects in regards to DNA damage reduction (50% or above) when compared to before supplementation. According to Stracke et al. [33], supplementing 200 g of slightly boiled carrot (carotenoids 23-24 mg/d) every day for 2 weeks had no affect on DNA strand breaks. In a previous study that evaluated the carotenoid content, the all-trans-β-carotene content, which is the most active provitamin, was shown to increase slightly after boiling in water. However, a significant reduction in the level of DNA damage when 330 ml (carotenoids 38 mg/d) was supplemented in the form of juice for 2 weeks observed by Pool-Zobel et al. [34]. The amount of carrot juice supplement used in this research was identical to 20.49 mg of β-carotene, which was lower than the doses evaluated in the above studies; however, a significant reduction in DNA damage was still observed after supplementation. It has been reported [35], that the activity of all-trans-β-carotene is reduced with storage, so short-term storage is desirable. The carrot provided in this research was squeezed for delivery and consumed as fresh juice on the same day, which may have contributed to its ability to recover DNA damage.
No significant differences in erythrocyte catalase, glutathione peroxidase (GSH-Px), and SOD (superoxide dismutase) analysis, which evaluate ROS removal antioxidant enzymes acting on the antioxidant defense system, were observed before and after supplementation. In a previous study [36], which evaluated the effect of red wine supplementation in healthy adults for 7 days, the red blood cell catalase and SOD activation were shown to significantly increase, but there was no significant changes in the activation of GSH-Px. Nielsen et al. [37] also reported that SOD activation significantly increased, but catalase and GSH-Px did not show significant changes, when healthy adults consumed parsley for 2 weeks. It was also reported that antioxidant enzymes activation in red blood cells was not improved in healthy adults after supplementation with fruit and vegetable [38]. In addition, supplementation of grape juice to smokers for 8 weeks was shown to increase catalase, but SOD and GSH-Px did not change [39]. It is not easy to interpret the meaning of the increase or decrease of antioxidant enzymes in this nutrition intervention study. This is the case because the human body works to maintain the physiological homeostasis, and many antioxidant enzymes are involved and used up when the oxidizing stress is high, but in some cases it may increase the generation of antioxidant enzymes. Therefore, the activation of antioxidant enzymes may not be a sensitive biomarker of the antioxidant nutrition status in the human body and therefore other surrogate markers of oxidative stress must be used.
It is important to improve the profiles of lipid in the blood, because when ROS is increased in the human body as is the case for smokers, it oxidizes not only DNA but, also, the lipid and lipoprotein in the blood. This will ultimately damage endotheliocytes due to oxidization of LDLs, which is a major factor of cardiovascular diseases. The baseline blood plasma lipid levels were within the normal range for the smokers in all the groups, and all the TG, TC, HDL-C, and LDL-C levels did not change before and 8 weeks after supplementation with carrot juice, β-carotene, and placebo. These results were consistent with the results of a previous study. In this previous study, no changes in the blood plasma lipid levels were observed when isoflavone supplementation was conducted for 8 weeks in healthy woman during menopause [40]. In addition, Hininger et al. [41] reported that there was no change in the blood plasma lipid levels in smokers supplementing carrot, tomato, and spinach. Also, it has been reported [42] that there was no change in the blood plasma lipid level in healthy female adults after supplementing tomato juice for 2 weeks. It seems that more studies are needed to better understand the effects of vegetable and fruit supplementation on blood plasma lipid levels.
In this study, DNA damage, which is a major problem in smokers, was effectively improved with supplementation of carrot juice and refined antioxidant vitamin, and both nutrition intervention methods produced similar degrees of effectiveness. Thus, supplementation of carrot juice as a food, which contains ample antioxidant nutrients including vitamin C, may be used to prevent diseases caused by oxidized damage in smokers.
References
1. Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993; 90:7915–7922. PMID: 8367443.

2. Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci. 1993; 686:12–27. PMID: 8512242.

3. Riso P, Martini D, Visioli F, Martinetti A, Porrini M. Effect of broccoli intake on markers related to oxidative stress and cancer risk in healthy smokers and nonsmokers. Nutr Cancer. 2009; 61:232–237. PMID: 19235039.

4. Wang MY, Lutfiyya MN, Weidenbacher-Hoper V, Anderson G, Su CX, West BJ. Antioxidant activity of noni juice in heavy smokers. Chem Cent J. 2009; 3:13. PMID: 19807926.

5. Riso P, Martini D, Møller P, Loft S, Bonacina G, Moro M, Porrini M. DNA damage and repair activity after broccoli intake in young healthy smokers. Mutagenesis. 2010; 25:595–602. PMID: 20713433.

6. van den Berg R, van Vliet T, Broekmans WM, Cnubben NH, Vaes WH, Roza L, Haenen GR, Bast A, van den Berg H. A vegetable/fruit concentrate with high antioxidant capacity has no effect on biomarkers of antioxidant status in male smokers. J Nutr. 2001; 131:1714–1722. PMID: 11385058.

7. Jacobson JS, Begg MD, Wang LW, Wang Q, Agarwal M, Norkus E, Singh VN, Young TL, Yang D, Santella RM. Effects of a 6-month vitamin intervention on DNA damage in heavy smokers. Cancer Epidemiol Biomarkers Prev. 2000; 9:1303–1311. PMID: 11142415.
8. Lee BM, Lee SK, Kim HS. Inhibition of oxidative DNA damage, 8-OHdG, and carbonyl contents in smokers treated with antioxidants (vitamin E, vitamin C, β-carotene and red ginseng). Cancer Lett. 1998; 132:219–227. PMID: 10397477.

9. Møller P, Viscovich M, Lykkesfeldt J, Loft S, Jensen A, Poulsen HE. Vitamin C supplementation decreases oxidative DNA damage in mononuclear blood cells of smokers. Eur J Nutr. 2004; 43:267–274. PMID: 15309445.

10. Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Palmgren J, Freedman LS, Haapakoski J, Barrett MJ, Pietinen P, Malila N, Tala E, Liippo K, Salomaa ER, Tangrea JA, Teppo L, Askin FB, Taskinen E, Erozan Y, Greenwald P, Huttunen JK. α-Tocopherol and β-carotene supplements and lung cancer incidence in the α-tocopherol, β-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996; 88:1560–1570. PMID: 8901854.
11. Database on the isoflavone content of foods. USDA-Iowa State University [Internet]. cited 2003 April 10. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=6382.
12. Kim JS, Yoon S. Isoflavone contents and β-glucosidase activities of soybeans, Meju, and Doenjang. Korean J Food Sci Technol. 1999; 31:1405–1409.
13. Hertog MGL, Hollman PCH, Katan MB. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J Agric Food Chem. 1992; 40:2379–2383.

14. Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993; 342:1007–1011. PMID: 8105262.

15. Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S, Pekkarinen M, Simic BS, Toshima H, Feskens EJ, Hollman PC, Katan MB. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med. 1995; 155:381–386. PMID: 7848021.
16. Bilyk A, Cooper PL, Sapers GM. Varietal differences in distribution of quercetin and kaempferol in onion (Allium cepa L.) tissue. J Agric Food Chem. 1984; 32:274–276.

17. Bilyk A, Sapers GM. Distribution of quercetin and kaempferol in lettuce, kale, chive, garlic chive, leek, horseradish, red radish, and red cabbage tissues. J Agric Food Chem. 1985; 33:226–228.

18. Park YK, Kim Y, Park E, Kim JS, Kang MH. Estimated flavonoids intake in Korean adults using semiquantitative foodfrequency questionnaire. Korean J Nutr. 2002; 35:1081–1088.
19. Kim M, Kim MC, Park JS, Park EJ, Lee JO. Determination of antioxidants contents in various plants used as tea materials. Korean J Food Sci Technol. 1999; 31:273–279.
20. Rojas E, Lopez MC, Valverde M. Single cell gel electrophoresis assay: methodology and applications. J Chromatogr B Biomed Sci Appl. 1999; 722:225–254. PMID: 10068143.

21. Aebi H. Bergmeyer HU, editor. Catalase. Methods of Enzymatic Analysis. 1974. Weinheim: Verlag Chemie;p. 673–678.

22. Beutler E. Glutathione peroxidase. Red Cell Metabolism: A Manual of Biochemical Methods. 1984. Orlando: Grune and Stratton;p. 71–73.
23. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974; 47:469–474. PMID: 4215654.

24. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499–502. PMID: 4337382.

25. Hemilä H, Kaprio J. Modification of the effect of vitamin E supplementation on the mortality of male smokers by age and dietary vitamin C. Am J Epidemiol. 2009; 169:946–953. PMID: 19218294.
26. Li N, Jia X, Chen CY, Blumberg JB, Song Y, Zhang W, Zhang X, Ma G, Chen J. Almond consumption reduces oxidative DNA damage and lipid peroxidation in male smokers. J Nutr. 2007; 137:2717–2722. PMID: 18029489.

27. Lee J, Lee E, Oh E, Lee J, Sul D, Kim J. Increased DNA damage of lymphocytes in Korean male smokers. J Prev Med Public Health. 2007; 40:16–22. PMID: 17310594.

28. Yildiz A, Gür M, Yilmaz R, Demirbağ R, Celik H, Aslan M, Koçyiğit A. Lymphocyte DNA damage and total antioxidant status in patients with white-coat hypertension and sustained hypertension. Turk Kardiyol Dern Ars. 2008; 36:231–238. PMID: 18765966.
29. Zeyrek D, Cakmak A, Atas A, Kocyigit A, Erel O. DNA damage in children with asthma bronchiale and its association with oxidative and antioxidative measurements. Pediatr Allergy Immunol. 2009; 20:370–376. PMID: 18801009.

30. Gill CI, Haldar S, Boyd LA, Bennett R, Whiteford J, Butler M, Pearson JR, Bradbury I, Rowland IR. Watercress supplementation in diet reduces lymphocyte DNA damage and alters blood antioxidant status in healthy adults. Am J Clin Nutr. 2007; 85:504–510. PMID: 17284750.

31. Park YK, Lee SH, Park E, Kim JS, Kang MH. Changes in antioxidant status, blood pressure, and lymphocyte DNA damage from grape juice supplementation. Ann N Y Acad Sci. 2009; 1171:385–390. PMID: 19723080.

32. Schneider M, Diemer K, Engelhart K, Zankl H, Trommer WE, Biesalski HK. Protective effects of vitamins C and E on the number of micronuclei in lymphocytes in smokers and their role in ascorbate free radical formation in plasma. Free Radic Res. 2001; 34:209–219. PMID: 11264897.

33. Stracke BA, Rüfer CE, Bub A, Briviba K, Seifert S, Kunz C, Watzl B. Bioavailability and nutritional effects of carotenoids from organically and conventionally produced carrots in healthy men. Br J Nutr. 2009; 101:1664–1672. PMID: 19021920.

34. Pool-Zobel BL, Bub A, Müller H, Wollowski I, Rechkemmer G. Consumption of vegetables reduces genetic damage in humans: first results of a human intervention trial with carotenoid-rich foods. Carcinogenesis. 1997; 18:1847–1850. PMID: 9328185.

35. Kim HY, Lim YI, Russell RM. Changes in carotenoids contents in pureed and cooked carrot and spinach during storage. Korean J Soc Food Cookery Sci. 2003; 19:83–95.
36. Fernández-Pachón MS, Berná G, Otaolaurruchi E, Troncoso AM, Martín F, García-Parrilla MC. Changes in antioxidant endogenous enzymes (activity and gene expression levels) after repeated red wine intake. J Agric Food Chem. 2009; 57:6578–6583. PMID: 19722566.

37. Nielsen SE, Young JF, Daneshvar B, Lauridsen ST, Knuthsen P, Sandström B, Dragsted LO. Effect of parsley (Petroselinum crispum) intake on urinary apigenin excretion, blood antioxidant enzymes and biomarkers for oxidative stress in human subjects. Br J Nutr. 1999; 81:447–455. PMID: 10615220.

38. Freese R, Dragsted LO, Loft S, Mutanen M. No effect on oxidative stress biomarkers by modified intakes of polyunsaturated fatty acids or vegetables and fruit. Eur J Clin Nutr. 2008; 62:1151–1153. PMID: 17671440.
39. Park YK, Park E, Kim JS, Kang MH. Daily grape juice consumption reduces oxidative DNA damage and plasma free radical levels in healthy Koreans. Mutat Res. 2003; 529:77–86. PMID: 12943921.

40. Hall WL, Vafeiadou K, Hallund J, Bugel S, Reimann M, Koebnick C, Zunft HJ, Ferrari M, Branca F, Dadd T, Talbot D, Powell J, Minihane AM, Cassidy A, Nilsson M, Dahlman-Wright K, Gustafsson JA, Williams CM. Soy-isoflavone-enriched foods and markers of lipid and glucose metabolism in postmenopausal women: interactions with genotype and equol production. Am J Clin Nutr. 2006; 83:592–600. PMID: 16522905.

41. Hininger I, Chopra M, Thurnham DI, Laporte F, Richard MJ, Favier A, Roussel AM. Effect of increased fruit and vegetable intake on the susceptibility of lipoprotein to oxidation in smokers. Eur J Clin Nutr. 1997; 51:601–606. PMID: 9306086.

Fig. 1
Changes in the degree of lymphocyte DNA damage expressed as Tail moment before and after supplementation for 8wks. Significantly different from week 0 and week 8, **P < 0.01, ***P < 0.001
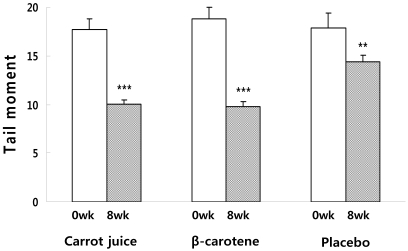
Fig. 2
Changes in the degree of lymphocyte DNA damage expressed as DNA in tail (%) before and after supplementation for 8wks. Significantly different from week 0 and week 8, ***P < 0.001
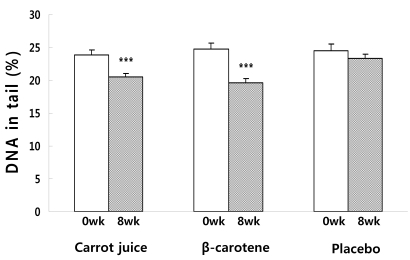
Fig. 3
Changes in the degree of lymphocyte DNA damage expressed as Tail length (um) before and after supplementation for 8wks. Significantly different from week 0 and week 8, ***P < 0.001
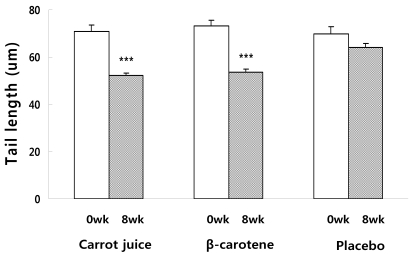
Table 2
Anthropometric indices, smoking and drinking habits of the subjects
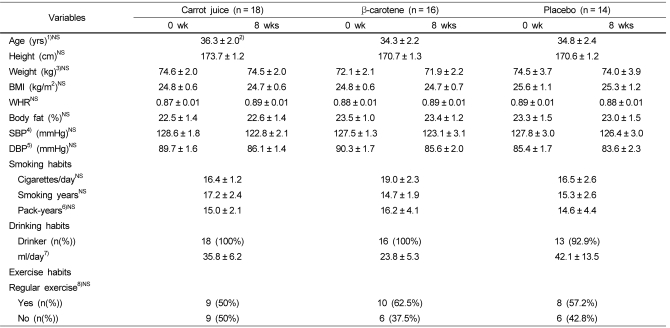
1)All values are means ± SE.
2)No significant differences among three groups analyzed by ANOVA
3)No significant differences within each group analyzed by paired t-test
4)SBP, systolic blood pressure
5)DBP, diastolic blood pressure
6)pack-years = (Cigarettes smoked/day × years smoked)/20
7)One drink is a dose of alcoholic beverage that delivers half ounce of pure alcohol (1 drink = 8-12 oz of beer 1 oz of hard liquor).
8)Significance as determined by χ2 test, P-value less than 0.05
Table 3
Daily intake of nutrients at 0 and 8 weeks after carrot juice, β-carotene and placebo supplementation




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download