Abstract
Monoclonal antibodies (MAbs) are widely applied in disease diagnoses. Herein, we report a MAb, WF-4, against Influenza A virus nucleoprotein (NP), its broad response with Influenza A virus, and its application in an immunohistochemistry (IHC) assay. WF-4 was screened by immunofluorescence assay (IFA). The results showed that its reactivity with baculovirus-expressed full-length recombinant NP (rNP) in Western blot (WB), indicating its IHC applicability. Fifteen Influenza A virus (reference subtypes H1 to H15) infected chicken embryonated chorioallantoic membranes (CAM), fixed by formalin, were all detectable in the WF-4-based IHC assay. Also, the reactivity of the IHC test with NP from experimentally inoculated H6N1 and from all recent outbreaks of H5 subtype avian Influenza A virus (AIV) field cases in Taiwan showed positive results. Our data indicate that CAM, a by-product of Influenza A virus preparation, is helpful for Influenza A virus-specific MAb characterization, and that the WF-4 MAb recognizes conserved and linear epitopes of Influenza A virus NP. Therefore, WF-4 is capable of detecting NP antigens via IHC and may be suitable for developing various tests for diagnosis of Influenza A virus and, especially, AIV infection.
Influenza viruses, members of the Orthomyxoviridae family, are enveloped, and the genome consists of six to eight segments of linear, negative-sense, single-stranded ribonucleic acid (RNA). Three types of influenza viruses, A, B, and C, have been classified based on antigenic differences in their nucleoprotein (NP) and matrix protein. Influenza A virus carrying eight RNA segments is further divided into subtypes based on the antigenic nature of their hemagglutinin (HA) and neuraminidase (NA) surface glycoproteins [11]. Sixteen HA (H1–H16) and nine NA (N1–N9) subtypes have been identified in aquatic birds to date [617]. Avian Influenza A virus (AIV) naturally infects a variety of avian species, as well as humans and several mammalian species such as pigs and horses [111]. Based on the severity of the clinical signs and the mortality rate in experimentally inoculated chickens, AIV can be categorized as highly pathogenic AIV (HPAIV) or low pathogenic AIV (LPAIV). To date, only certain H5 and H7 isolates have been characterized as HPAIV in chickens [216]. Because AIV can potentially cause a devastating viral disease in poultry and can have a high impact on animal and human health, it has become a matter of increasing public concern lately [18]. There is a compelling global need to be well-equipped with diagnostic tools to survey the prevalence of AIV, not only HPAIV, due to the rapid evolution of AIV and its ability to acquire pathogenicity for chickens when the virus is established in the poultry industry [415]. Hence, the optimal diagnostic tool should be capable of detecting all subtypes of AIV during the asymptomatic phase of initial infection so that precautionary and preventive measures can be implemented in a timely manner by veterinary authorities in order to cease viral circulation and evolution in domestic avian and mammalian populations.
Given its high sequence conservation and abundance in the virion [11], NP is a perfect marker for all influenza viruses. To examine the presence of specific infectious agents, such as AIV, monoclonal antibody (MAb) assessment is a preferred diagnostic tool because of MAb specificity, the unlimited availability of identical antibodies, and the ease of standardization of a MAb-based assay. Therefore, the application of MAb against the NP of AIV could be highly useful for detecting the presence of AIV. In this study, we present an NP-specific MAb against the H5N2 LPAIV isolated from the earliest epidemic of a poultry case in Taiwan in 2003 [4]. In addition, the results of detecting fifteen subtypes (H1–H15) of Influenza A virus on chorioallantoic membranes (CAMs), the by-product of influenza virus preparation, and the etiological diagnosis of domestic field cases via the NP MAb-based immunohistochemistry (IHC) assay are presented. The results indicate the validity and suitability for monitoring the health of poultry populations of applying the MAb-based IHC assay in routine pathological examinations or for screening during disease surveillance.
The LPAIV H5N2 (A/Ck/Taiwan/1209/03, H5N2/1209, GenBank accession No. AY573918) isolated from apparently healthy chickens in Taiwan in 2003 [4] was the source of the antigen used for mouse immunization and Western blot (WB) analysis in this study. Briefly, 10-day-old embryonated specific pathogen-free (SPF) chicken eggs (Animal Health Research Institute [AHRI], Taiwan) were inoculated via the allantoic cavity with H5N2/1209 inoculum. The allantoic fluid was collected, inactivated by 0.2% formaldehyde for 24 h, clarified by centrifugation at 2,000 × g for 15 min, and further concentrated at 70,000 × g centrifugation for 2 h. The viral pellet was resuspended with 1/100 original volume of 0.08% NaN3-phosphate-buffered saline (PBS) and tested by HA test as described in the Avian influenza chapter of the World Organisation for Animal Health (OIE) Manual 2015 [19]. The protein concentration of the viral suspension was also determined. The A/Dk/Yunlin/04 (H5N2) isolate was chosen as the source of the sequence origin of baculovirus-expressed recombinant NP (rNP). The A/wild Dk/Tainan/1634/09 (H1N1), A/Dk/Tainan/A30/02 (H5N2), H5N2/1209, A/wild Dk/830/05 (H5N2), A/Ck/Miaoli/2904/00 (H6N1), A/Ck/Changhua/7-5/99 (H6N1), and A/Dk/Tainan/A45/03 (H7N7) were isolated from either wild birds or domestic poultry birds in Taiwan. Those isolates, together with A/Dk/HongKong/820/80 (H5N3) from Dr. Hiroshi Kida, Hokkaido University, Sapporo, Japan, were propagated in embryonated SPF eggs as antigens for WB analysis and experimental inocula as mentioned above. Fifteen Influenza A virus reference subtypes (Table 1) were provided by Dr. Kida to prepare viral-infected CAMs for IHC examination. Newcastle disease virus (NDV) 060901, as a negative control, was isolated from poultry in Taiwan. The 50% egg infectious dose (EID50) of A/Ck/Changhua/7-5/99 (H6N1) allantoic fluid for experimental infection was determined.
The H5N2 (A/duck/Yunlin/04) full-length NP was amplified by reverse transcription-polymerase chain reaction (PCR) with forward primer NP-F: 5′-ATGGCGTCTCAAGGCACCAAAC-3′ and reverse primer NP-R: 5′-TTAATTGTCATACTCCTCTGCATTGTC-3′. The NP full-length gene was cut from the T&A Cloning Vector (Yeastern Biotech, Taiwan) and subcloned in a frame into the corresponding pFastBac-HT-C plasmid (Invitrogen, USA). Additionally, a 6 histidine residue was designed to tag on the 3′ end of the NP by engineering the Spe I site embedded reverse primer NP-HisSpel-R: 5′-AGCACTAGTTCAGTGGTGGTGGTGGT-3′ in company with the EcoRI site embedded forward primer NP-EcoR-F: 5′-AGGAATTCGAATGGCGTCTCAAGGCAC-3′ to obtain the PCR product and used to construct the rNP vector. Subsequently, the recombinant vector was transposed into the baculovirus (Autographa californica) genome to form NP pFastBac recombinant bacmids. Positive recombinant bacmids were used to transfect an insect cell line (Spodofera frugiperda, Sf9) for viral particle formation. All procedures for viral particle production were performed according to the manufacturer's manual (Bac-to-Bac; Invitrogen). Recombinant NP from baculovirus-infected Sf9 cells was obtained, purified by Ni-NTA Agarose (Invitrogen), and used as an antigen for WB analysis.
Twelve-week-old female BALB/c mice were subcutaneously immunized three times each with 50 µg (211 HA U/4.61 mg/mL) of inactivated H5N2/1209 at two-week intervals. Immunogen of inactivated virus was mixed with complete Freund's adjuvant for priming and incomplete Freund's adjuvant for the next two boosters. The final booster of 50 µg/0.1 mL inactivated virus in PBS was given to anesthetized mice via an intrasplenic route [9] three days before fusion. The splenocytes from mice showing the highest immunofluorescence assay (IFA) titers were fused with myeloma cells Sp2/0-Ag14 (ATCC CRL-1851) to produce hybridomas according to a standard protocol [8]. Positive hybridomas were screened by indirect IFA on H5N2/1209 plates and WB (both described below). After three rounds of subcloning, selected clones were inoculated intraperitoneally into incomplete Freund's adjuvant-primed BALB/c mice to produce ascitic fluid containing MAb. The hybridoma cultural supernatant (cul. sup.) and ascitic fluid were collected for subsequent characterization. The isotype of MAb from the hybridoma cul. sup. was analyzed by IsoStrip Isotyping Kit (Roche Diagnostics, USA) according to the manufacturer's recommendations. The care and use of the mice were approved by the Institutional Animal Care and Use Committee, National Taiwan University, Taiwan, to ensure compliance with local legal and ethical requirements.
An MDCK cell suspension (100 µL) containing 3 × 104 cells was added to each well of a 96-well plate. Following overnight incubation, the 80% confluent monolayers were infected by 10 TCID50/100 µL/well H5N2/1209 in C-DMEM (DMEM supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, 2 µg/mL trypsin, BSA 0.2%, HEPES 20 mM) and incubated for 22 h. Subsequently, the plates were fixed with 70% (v/v) cold acetone for 20 min and then rinsed by PBS once, air dried, and stored at −20℃. Fifty microliters of hybridoma cul. sup. per well were added to the plate and the plate was incubated at room temperature for 2 h. The cells in each well were then washed with PBS and stained with 50 µL of fluorescein-conjugated goat anti-mouse IgG, IgA, and IgM (ICN Pharmaceuticals, USA) at a 1:1,000 dilution at room temperature for 1 h. After washing, IFA results were examined under a fluorescence microscope.
The AIV concentrated allantoic fluid and purified rNP were denatured in sample buffer (125 mM Tris-base, 2 mM EDTA, 2% SDS, and 5% β-mercaptoethanol, pH 6.8) by boiling for 10 min. Denatured proteins were separated in 10% SDS-PAGE gel at 100 V for 3 h. The proteins were then transferred to an NC membrane (Bio-Rad, USA), and the blots were blocked with 10% (w/v) skim milk for 2 h at room temperature. Subsequently, the blots were incubated with anti-NP MAb (WF-4 cul. sup. diluted 5-fold) for 1 h at room temperature and then washed three times with washing buffer (1× PBS and 1% Tween 20, pH 7.4). The HRP-conjugated goat anti-mouse IgG (1:2,000 × diluted; Jackson ImmunoResearch Laboratories Inc., USA) was added and incubated for 1 h at room temperature. After washing, the membrane was developed with TMB (Kirkegaard and Perry Laboratories, USA) for 15 min at room temperature. The reaction was stopped by rinsing with distilled water.
The CAMs from fifteen eggs infected with the Influenza A virus reference subtypes and having allantoic fluids with HA activity were collected [3] as the NP source for IHC assays to mimic that of the formalin-fixed tissue for histopathological examination. In brief, eleven-day-old embryonated chicken eggs were inoculated with 0.2 mL of stock Influenza A virus reference subtypes and incubated at 37℃ for 48 h before being removed from the incubator and chilled at 4℃ overnight. After the air sac end of the eggshell was cut off with scissors, the allantoic fluid was carefully collected, using an 18 G syringe, to avoid bleeding. The embryo and its attachments were discarded. The CAM was left adhered to the eggshell wall. The HA titer of the allantoic fluid was determined as described in the OIE Manual [19]. The CAMs having allantoic fluid with HA activity were removed with forceps, fixed in 10% neutral buffered formalin, and processed for IHC examination.
Histopathological sample blocks from H6N1 experimentally infected cases, field poultry cases, and CAMs for IHC were sectioned (3 µm thick). Tissue sections were placed onto microscope slides SuperFrost Plus (Thermo Fisher Scientific Inc, USA) [l], deparaffinized in xylene, and rehydrated in graded alcohol and distilled water. The sections were treated with 100 µg/mL of proteinase K (Roche Diagnostics, Germany) in 0.6 mol/L of Tris (pH 7.5)/0.1% CaCl2 for 15 min. Endogenous peroxidase activity and non-specific antigens were blocked with 3% hydrogen peroxidase in methanol (Merck, Germany) and PowerBlock solution (BioGenex Laboratories, USA) for 10 min each. Tissue sections were then incubated at room temperature for 60 min with MAb WF-4. The detection system was sequentially conducted with Super Enhancer reagent (BioGenex Laboratories) incubation for 30 min and with Polymer-HRP reagent (BioGenex Laboratories) incubation for 40 min at room temperature. The aminoethylcarbazole (AEC; Dako, Denmark) was the substrate chromogen, and hematoxylin was used as a counterstain. The tissues from SPF and influenza virus-inoculated chickens served as negative and positive controls, respectively.
Twelve six-week-old SPF white leghorn chickens were divided into four groups for experimental inoculation by A/Ck/Changhua/7-5/99 (H6N1) as shown in Table 2. Briefly, Groups A and D had 2 chickens each, and Groups B and C had 4 chickens each. Chickens in Groups A, B, and C were inoculated with 106 EID50/0.1 mL H6N1 via intravenous, intranasal, and intraocular routes, respectively. Group D was the sham control. Half of the chickens in each group were sacrificed at 3 days post-inoculation (DPI), and the other half, at 5 DPI. Brain tissue from chickens experimentally infected by Taiwan NDV isolate ND060901, which causes encephalitis similar to that induced by H6N1, was the negative control for the WF-4 IHC test. The care and use of chickens were approved by the Institutional Animal Care and Use Committee, AHRI, Council of Agricultural Executive Yuan (Taiwan) to ensure compliance with local legal and ethical requirements.
Field cases of HPAIV infection from chicken farms collected in 2012, from chicken, goose, duck, and turkey farms collected in 2015, and a duck carcass index case in 2017 were included in this study. All routine pathological and virological examinations, including sequencing for all cases mentioned above, were performed at AHRI (Taiwan).
The immunized and euthanized BALB/c mice showed an IFA titer at the minimal 400× dilution of serum when it was sacrificed on fusion day. Thirty-four MAbs were obtained by sequential cloning and screening by IFA from the IFA-positive parental hybridoma. The MAb WF-4 used in this study displayed a band with a molecular weight of about 56 kDa, similar to that of AIV NP, on WB analysis and was further characterized by WB with full-length rNP expressed by baculovirus (panel A in Fig. 1). The rNP band had an expected molecular weight of about 61 kDa, unlike that of the purified H5N2/1209 on the blot (panel A in Fig. 1).
All viral isolates from wild birds and domestic poultry, including subtypes H1, H5, H6, and H7, exhibited band sizes similar to that of H5N2/1209 on WB (panel B in Fig. 1).
At low magnification, brownish-red epithelial lining cells with flattened to cuboidal shapes were observed in the single layer allantoic membranes of all CAMs infected with one of the fifteen reference serotypes of Influenza A virus. Representative sample results are shown in Fig. 2. At higher magnification, positive signals in the cytoplasm of the epithelium of allantoic endoderm with pyknosis and amorphous debris were noted. Some red-brownish deposits were observed in the nuclear area. The NP antigen was not found in the ectoderm or mesoderm.
One chicken from Group A that was found dead at 3 DPI had swollen kidneys and heavy urate deposition on serosal surfaces throughout the coelomic cavity. The other chicken from Group A, sacrificed at 3 DPI, demonstrated similar gross signs as the dead chicken. The chickens from the other groups were all clinically healthy and grossly normal, except one; chicken No. 3 in Group B, sacrificed at 5 DPI, had swollen kidneys and gross lesions but moderate urate deposition. This chicken presented strong IHC-positive results in the renal parenchyma (panel A in Fig. 3). Scattered degeneration, necrosis, and regeneration of the renal tubular epithelium were observed (panel B in Fig. 3). The varying amount of deposition of the positive signal representing the influenza viral NP corresponded to the degenerative to necrotic process in the renal tubule (panel C in Fig. 3). The other chicken (No. 4) from Group B, also sacrificed at 5 DPI, was grossly normal but showed moderate kidney lesions and a positive IHC signal like chicken No. 3 (data not shown). The other chickens from Groups C and D all showed IHC-negative results with WF-4 (data not shown). Brain tissue from chickens infected with NDV isolate 060901 did not present positive IHC signals with WF-4 (data not shown). The field cases from 2012 were from an H5N2 HPAIV infection in poultry farms affecting layer, broiler breeder, and native chickens. Those in 2015 were from H5N2, H5N3, and H5N8 HPAIV infections affecting goose, chicken, turkey, and duck farms. We also examined a duck H5N6 HPAIV index case from 2017. The results of IHC staining were similar in all naturally infected cases. IHC-positive signals were demonstrated in all organs, such as larynx (panel D in Fig. 3), heart (panel E in Fig. 3), brain, lung, kidney, pancreas, and integumen. Interestingly, IHC-positive signal strengths were highest in the heart muscle fiber of a 2015 H5N8 infection case (panel E in Fig. 3) and the endothelial cell lining of the spleen and liver of a 2015 H5N2 case (panel F in Fig. 3). In addition, there were IHC-positive signals in goose ovary (panel G in Fig. 3) of an H5N3 infection in 2015 and in duck brain (panel H in Fig. 3) of a 2017 H5N6 infection.
First, the WB results indicated that the NP epitope recognized by WF-4 should be linear and conserved among the AIVs detected. After that, the capability of WF-4 to reveal the NP antigen in IHC was checked on fifteen reference subtypes of Influenza A virus, H1 to H15, which were isolated from 1934 to 1983 from worldwide sources (Table 1), representing broad coverage of the viral isolates.
To obtain separate tissue specimens with the 15 reference subtypes, chicken embryonated CAMs infected with individual subtypes of Influenza A virus for the standard immunodiffusion assay [3] were sampled. Because CAM from a chicken embryo possesses the tissue tropism for Influenza A virus, CAM was chosen as the tissue to mimic the target object of Influenza A virus for IHC examination. The CAMs obtained from the embryo chicken eggs inoculated with fifteen Influenza A virus reference subtypes all showed positive IHC results if their allantoic fluid had an HA titer when they were collected at 2 DPI. But an absence or detachment of allantoic epithelial cells from the CAM endoderm, where the inoculated virus mainly replicates, was occasionally noticed during IHC examination (data not shown). A possible explanation of this occurrence is the unequal susceptibility of the allantoic epithelium to different reference subtypes or variable subtypic pathogenicity to the chicken embryo when the CAM was collected at the same DPI. However, the feasibility of using CAM from Influenza A virus-inoculated chicken embryo as the reference Influenza A virus subtype for the IHC test is evident from the results. They confirm that the WF-4 IHC assay can detect all Influenza A virus reference subtypes (Fig. 2), including isolates H1 and H2 subtypes of non-avian origin. Therefore, a blocking or competitive enzyme-linked immunosorbent assay assembled with a combination of WF-4 and baculovirus-expressed NP may potentially detect the anti-NP antibody from all Influenza A virus-infected animals.
Immunofluorescence (IF) staining, which has been widely used to localize antigen and antibody complexes in diagnostic laboratories since its first description [5], was applied to the screening of MAb in this study. IHC, the analogous assay of IF for demonstrating a specific antigen recognized by an antibody, has also shown strong competence for diagnostic purposes. Therefore, the feasibility of applying WF-4 to IHC was checked by examining the confirmed AIV cases from experimental inoculation and field outbreaks in Taiwan after IHC-positive results from all Influenza A virus reference isolate tests were obtained. Chickens experimentally inoculated with the early AIV isolate (subtype H6N1) isolated from domestic poultry in Taiwan were sampled. Furthermore, the latest H5N2 HPAIV isolate of AIV from 2012, which is likely to have evolved from the 2003 LPAIV, and the field cases from the 2015 HPAIV outbreak that heavily affected all poultry, especially geese, and the 2017 HPAIV index case were included in this test.
The histopathological lesions in the kidneys from chicken No. 3 inoculated by AIV H6N1 via the intranasal route, mimicking a natural infection, and sacrificed at 5 DPI were closely associated with the presence of an AIV NP signal revealed by IHC in situ. This chicken was clinically normal but, grossly, showed swollen kidneys with moderate urate deposition. Chicken No. 4 in the same group sacrificed on the same day showed no sign of illness and appeared grossly normal but showed moderate kidney lesions and IHC-positive signals under microscopic examination. The kidney gross lesions in this study are similar to those of the 1978 H6N1 outbreak case in chicken in Minnesota [7]. Also, the significant necrosis of renal tubular epithelial cells with IHC-positive signals in our experimental inoculation study, indicating the presence of an AIV NP antigen, corresponds to that from the chicken intravenously inoculated with a waterfowl-origin LPAIV H5N1 [14]. All of the above indicate that the chicken kidney is an important site for replication of LPAIV, which can be detected by WF-4 IHC during the asymptomatic phase of AIV infection. Therefore, WF-4-based IHC can be a useful tool for detecting newly introduced AIV with low pathogenicity in a poultry farm population, such as the H6N1 example shown in this study.
The H5N2 HPAIV isolates from chicken outbreaks in 2012 apparently originated from the H5N2 LPAIV in 2003, and the presence of this isolate has since been established in Taiwan. Genetic evidence suggested that these viruses were generated by reassortment of HA and NA genes derived from an American lineage 1994 Mexican-like H5N2 and other internal genes from the enzootic Eurasia lineage H6N1 [41215]. Positive IHC signals of WF-4 were evident in all tissues examined, including the larynx and comb, representative primary and secondary target sites of H5N2 HPAIV from 2012 field cases. Together with the positive WB results with H5N2 1209 and the early isolate of Taiwanese H5N2 LPAIV in 2003, these results indicate the competence of a WF-4-based IHC to reveal the virus during different stages of infection and phases of evolution from asymptomatic LPAIV to HPAIV.
The 2015 outbreaks of HPAIV H5N2, H5N3, and H5N8, which occurred mainly in western Taiwan, heavily affected all poultry farms, especially goose farms. Together with the index case of HPAIV H5N6 in 2017 in eastern Taiwan, those were novel virus infections in Taiwan and all have an HA gene derived from the Eurasia H5 clade 2.3.4.4 and were most likely introduced by migratory birds [1013]. They are different from the established American lineage H5N2 from 2003 in Taiwan. The WF-4-based IHC clearly demonstrated the same diagnostic results as those from the etiological and molecular diagnoses in the 2015 and 2017 incidences in this study. The diagnostic competence of performing WF-4-based IHC on AIV field cases is thus further validated.
The introduction and subsequent spread of AIV in domestic waterfowl may occur undetected, thereby increasing the risk of trans-species transmissions to highly susceptible chickens and mammals, including humans. Therefore, there is always a compelling need to survey for asymptomatic infections of any subtypic AIV in order to mitigate the potential risk of pathogenic evolution along the viral establishment, as well as to diagnose the case after an outbreak. The results presented in this study show that WF-4-based IHC assay is a suitable tool for fulfilling both purposes.
Figures and Tables
Fig. 1
Western blot analysis of monoclonal antibody WF-4. (A) Lane 1, molecular weight standard; Lane 2, H5N2/1209; Lane 3, baculovirus-expressed recombinant nucleoprotein. (B) Lane 1, molecular weight standard; Lane 2, A/wild duck/Tainan/1634/09 (H1N1); Lane 3, H5N2/1209; Lane 4, A/duck/Tainan/A30/02 (H5N2); Lane 5, A/wild duck/830/05 (H5N2); Lane 6, A/duck/Hong Kong/820/80 (H5N3); Lane 7, A/chicken/Miaoli/2904/00 (H6N1); Lane 8, A/duck/Tainan/A45/03 (H7N7).

Fig. 2
Twelve-day-old chicken embryo. (A) Normal chorioallantoic membrane (CAM). (B) Negative control for immunohistochemistry (IHC) assay. CAM infected by avian Influenza A virus reference subtypes and collected at 2 days post-inoculation. (C) H11N6. (D) H11N6. (E) H14N5. (F) H15N8. Ec, fibrous chorionic ectoderm; M, mesoderm; En, allantoic endoderm. H&E stain (A). IHC stain (B–F). Scale bars = 20 µm (D–F), 100 µm (C), 200 µm (A and B).
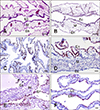
Fig. 3
Immunohistochemistry (IHC)- and H&E-stained tissue samples of experimentally infected chicken and poultry field cases. (A–C) Kidney from H6N1 experimentally infected via the intranasal route, clinically normal chicken at 5 days post-inoculation. (A) Multifocal IHC-positive signals densely distributed in the renal parenchyma. (B) Scattered degeneration, necrosis, and regeneration of renal tubular epithelium were observed in this H&E-stained kidney section. Spheroids of material clusters and calcification in the lumen of necrotic tubules were common. (C) Renal necrosis at different stages. (D) H5N2 highly pathogenic avian Influenza A virus (HPAIV) field cases in 2012. Lymphocytic laryngitis with necrosis of mucous gland cells (arrow). Avian Influenza A virus (AIV) nucleoprotein (NP) in individual epithelium. (E) Naturally infected with HPAIV H5N8 in goose, the influenza virus antigen is noted in the cardiac fibers. (F) Focal necrosis surrounded by hepatocytes with intranuclear and intracytoplasmic staining for AIV NP in chicken naturally infected with HPAIV H5N2 virus. The NP was immunolabeled at high levels in the endothelial cell lining of liver. (G) AIV antigens were identified in the primordial follicles and lymphocytes of the ovarian cortex. (H) There was specific immunolabeling in the nucleus and cytoplasm of neurons, glial cells, axons, and endothelial cells in duck naturally infected with HPAIV H5N6 virus. IHC stain (A, C–H). H&E stain (B). Scale bars = 20 µm (D and F), 50 µm (B, C, G, and H), 100 µm (E), 200 µm (A). a, normal tubular epithelium; b, pyknosis of tubular epithelial cells; c, karyorrhexis and heterophil infiltration; d, formation of urate tophi; e, normal; f, degeneration with minute IHC-positive staining in nucleus; g, necrotizing tubular cells with moderate IHC signals in both nucleus and cytoplasm; h, tubular cells with pyknosis covered by strong positive signals.
Table 1
Summary of Influenza A virus reference subtypes for the preparation of viral-infected chorioallantoic membrane and the hemagglutinin (HA) titer of the allantoic fluid
Acknowledgments
This work was supported by grants 101-2324-B-002-012, NSC 102-2622-B-002-012-CC2 of the National Science Council, Taiwan, R.O.C.; 106AS-9.1.1-HI-H5 and 107AS-8.1.1-HI-H5 of Animal Health Research Institute. The authors would like to thank Mr. Siou-Yu Lai for his excellent technical assistance.
References
1. Alexander DJ. A review of avian influenza in different bird species. Vet Microbiol. 2000; 74:3–13.


2. Alexander DJ. Orthomyxoviridae: avian influenza. In : Jordan FTW, Pattison M, Alexander DJ, Faragher T, editors. Poultry Diseases. 5th ed. London: W.B. Saunders;2002. p. 281–290.
3. Beard CW. Demonstration of type-specific influenza antibody in mammalian and avian sera by immunodiffusion. Bull World Health Organ. 1970; 42:779–785.


4. Cheng MC, Soda K, Lee MS, Lee SH, Sakoda Y, Kida H, Wang CH. Isolation and characterization of potentially pathogenic H5N2 influenza virus from a chicken in Taiwan in 2008. Avian Dis. 2010; 54:885–893.


5. Coons AH, Kaplan MH. Localization of antigen in tissue cells; improvements in a method for the detection of antigen by means of fluorescent antibody. J Exp Med. 1950; 91:1–13.


6. Fouchier RA, Munster V, Wallensten A, Bestebroer TM, Herfst S, Smith D, Rimmelzwaan GF, Olsen B, Osterhaus AD. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol. 2005; 79:2814–2822.



7. Halvorson DA, Karunakaran D, Newman JA. Avian influenza in caged laying chickens. Avian Dis. 1980; 24:288–294.

8. Harlow E, Lane D. Monoclonal antibodies. In : Harlow E, Lane D, editors. Antibodies: A Laboratory Manual. New York: Cold Spring Harbor Laboratory;1988. p. 139–244.
9. Hong TH, Chen ST, Tang TK, Wang SC, Chang TH. The production of polyclonal and monoclonal antibodies in mice using novel immunization methods. J Immunol Methods. 1989; 120:151–157.


10. Huang PY, Lee CD, Yip CH, Cheung CL, Yu G, Lam TT, Smith DK, Zhu H, Guan Y. Genetic characterization of highly pathogenic H5 influenza viruses from poultry in Taiwan, 2015. Infect Genet Evol. 2016; 38:96–100.

11. Lamb RL, Krug RM. Orthomyxoviridae: the viruses and their replication. In : Fields BN, Knipe DM, editors. Fields Virology. 1:4th ed. Philadelphia: Lippincott Williams & Wilkins;2001. p. 1216–1253.
12. Lee CCD, Zhu H, Huang PY, Peng L, Chang YC, Yip CH, Li YT, Cheung CL, Compans R, Yang C, Smith DK, Lam TTY, King CC, Guan Y. Emergence and evolution of avian H5N2 influenza viruses in chickens in Taiwan. J Virol. 2014; 88:5677–5686.



13. Lee MS, Chen LH, Chen YP, Liu YP, Li WC, Lin YL, Lee F. Highly pathogenic avian influenza viruses H5N2, H5N3, and H5N8 in Taiwan in 2015. Vet Microbiol. 2016; 187:50–57.


14. Slemons RD, Swayne DE. Replication of a waterfowl-origin influenza virus in the kidney and intestine of chickens. Avian Dis. 1990; 34:277–284.


15. Soda K, Cheng MC, Yoshida H, Endo M, Lee SH, Okamatsu M, Sakoda Y, Wang CH, Kida H. A low pathogenic H5N2 influenza virus isolated in Taiwan acquired high pathogenicity by consecutive passages in chickens. J Vet Med Sci. 2011; 73:767–772.


17. Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992; 56:152–179.



18. Webster RG, Hulse DJ. Microbial adaptation and change: avian influenza. Rev Sci Tech. 2004; 23:453–465.


19. World Organisation for Animal Health (OIE). Avian influenza (infection with avian influenza viruses). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Chapter 2.3.4. Paris: OIE;2015.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download