Abstract
Acknowledgments
References
Fig. 1
Histological classification of canine ovarian cysts. (A) Cysts of subsurface epithelial structure (SES): Oval structures located in the ovarian cortex surrounded by smooth muscle. (B) Cyst of SES: Lined by low epithelium without a basement membrane. (C) Follicular cyst (FC): Lined by granulosa cells with a “picket fence” appearance and persistence of recognizable theca interna with blood vessels. (D) FC: Lined by granulosa cells with a “picket fence” appearance and variably sized plaques of luteinized tissue as cystic content. (E) Cystic rete ovarii (CRO): Lined by a single cuboidal epithelium. (F) CRO: Lined by a single cuboidal ciliated epithelium. (G) Lutein cyst (LC): Lined by several layers of granulosa-lutein cells and a thin layer of fibrin on the luminal surface. Capsule of compressed ovarian stroma and vascular connective tissue. Central cavity contains residues of an oocyte (*). H&E stain (A–G). Scale bars = 100 µm (A, C, and E), 50 µm (B, D, and F), 300 µm (G).
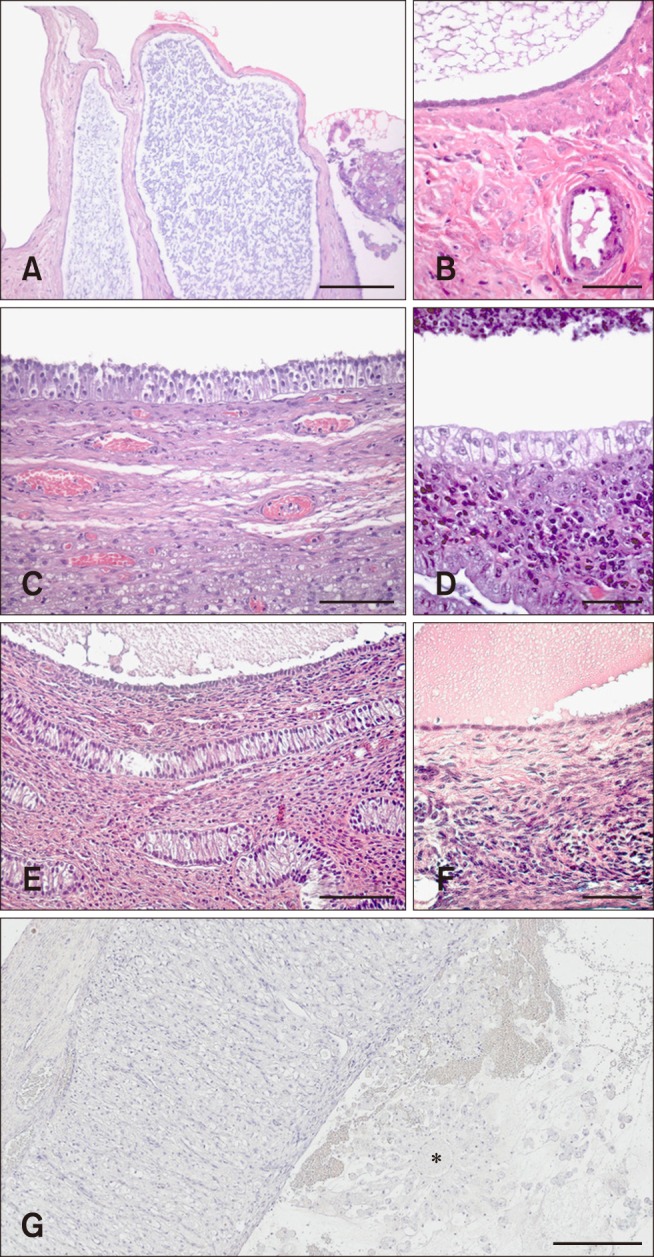
Fig. 2
Histological and immunohistochemical classifications of canine ovarian cysts. (A) Cysts of subsurface epithelial structure (SES): smooth muscle cells surrounding the low epithelium cells that line the cyst (*). (B) Cyst of SES: Low epithelium cells show a strong positive reaction to cytokeratin. (C) Follicular cyst (FC): cavity of the cyst (†). Elastic fibers surrounding the granulosa cells with a picket-fence appearance that lines the cyst. (D) FC: Lined by granulosa cells, which show no reaction to cytokeratin compared to cysts of SES and cystic rete ovarii (CRO). (E) CRO: Lined by cuboidal epithelium cells and surrounded by elastic fibers. (F) CRO: Lining epithelium cells show a weak positive reaction to cytokeratin. (G) Non-classifiable cyst (NCC): Smooth muscle cells and elastic fibers surround the low epithelium cells that line the cyst. (H) NCC: Lining epithelium cells show a positive reaction to cytokeratin. Elastica van Gieson stain (A, C, E, and G). Mouse anti-cytokeratin antibody stain (B, D, F, and H). Scale bars = 100 µm (A–H).
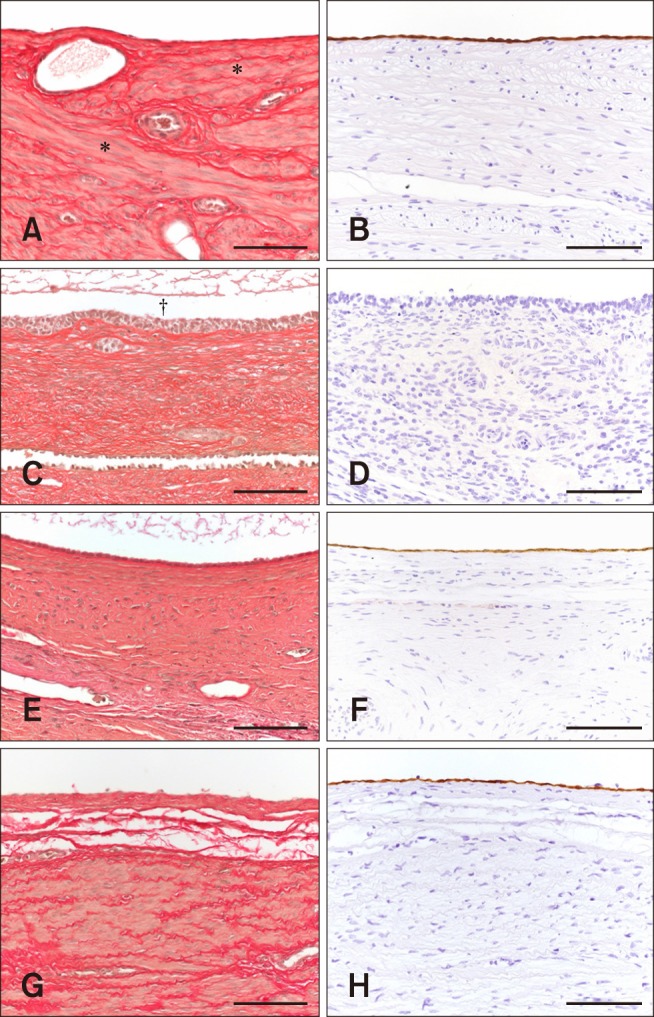
Table 2
Characteristics of individual bitches with ovarian cysts (OCs) that presented with gynecopathies
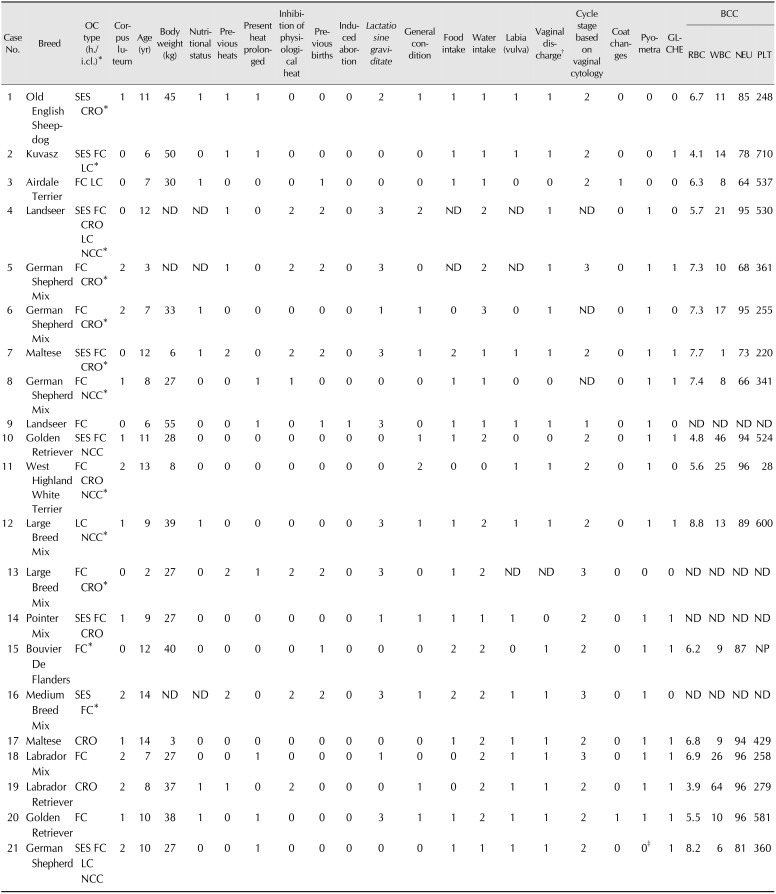
Corpus luteum: 0 = no, 1 = yes (single), 2 = yes (multiple); Nutritional status: 0 = normal, 1 = obese; Previous heats: 0 = regular, 1 = irregular, 2 = owner could not provide information (o.c.p.i.); Present heat prolonged: 0 = no, 1 = yes; Inhibition of physiological heat: 0 = no, 1 = yes, 2 = o.c.p.i.; Previous births: 0 = no, 1 = yes, 2 = o.c.p.i.; Induced abortion: 0 = no, 1=yes; Lactatio sine graviditate: 0 = no, 1 = regular, 2 = irregular, 3 = o.c.p.i.; General condition: 0 = good, 1 = moderate, 2 = poor; Food intake: 0 = inappetent, 1 = good, 2 = moderate; Water intake: 0 = nothing, 1 = normal, 2 = increased, 3 = multiplied; Labia (vulva): 0 = no edema, 1 = edema; Vaginal discharge: 0 = no, 1 = yes; Cycle stage based on vaginal cytology: 0 = anestrus, 1 = proestrus, 2 = estrus, 3 = metestrus; Coat changes: 0 = no, 1 = yes; Pyometra: 0 = no, 1 = yes; GLCHE: 0 = no, 1 = yes. Normal range: RBC, 5.5–8.5 T/L; WBC, 6–12 G/L; NEU, 59–79%; PLT, 200–460 G/L. h./i.cl., histological/immunohistochemical classification; SES, cysts of subsurface epithelial structure; CRO, cystic rete ovarii; FC, follicular cyst; LC, lutein cyst; NCC, non-classifiable cyst; ND, not determined; GLCHE, glandular cystic hyperplasia of the endometrium; BCC, blood cell count; RBC, red blood cells; WBC, white blood cells; NEU, neutrophils; PLT, platelets; NP, not possible. *In addition: OCs with a diameter > 1 cm were punctured (cyst fluid was analyzed for estrogen and progesterone concentration [18]). †Sero-sanguinous or sanguinous-purulent. ‡Mucometra.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


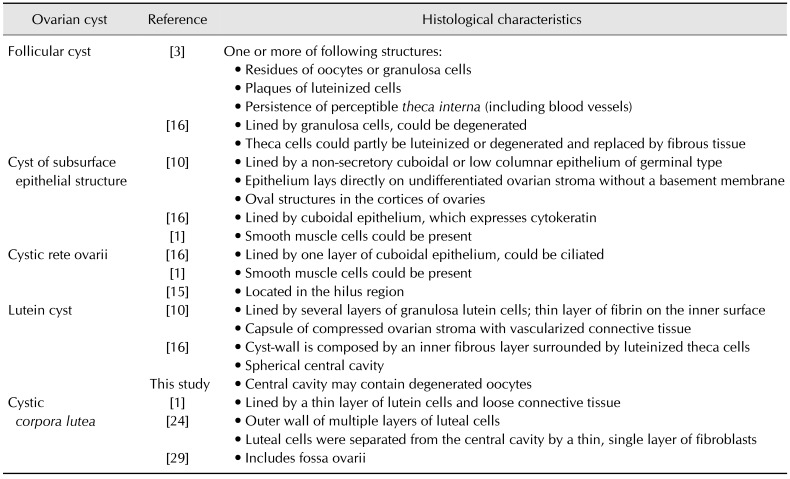
 XML Download
XML Download