Abstract
An effect of aging on cardiac morphology and function has been shown in humans. In horses, cardiac wall motion analysis using two-dimensional speckle tracking (2D-ST) has not yet been reported. Our study included 57 horses of different warmblood breeds between 3 and 30 years old. Age had a significant influence on left ventricular free wall (LVFW) systolic strain rate (p ≤ 0.05) and early diastolic relaxation (p ≤ 0.01). In the interventricular septum (IVS), systolic (p ≤ 0.01) and late diastolic (p ≤ 0.05) contraction velocities also increased with age. In our study, 2D-ST revealed important information on myocardial function, which was most evident in the LVFW, where measurements were highly reproducible. Aging seems to be associated with structural changes within the myocardium and with decreasing contraction capacity in old animals. These physiological, age-related processes should be considered when performing cardiac wall motion analysis of the 2D-ST results for the LVFW and IVS in horses.
So far, few studies have been published on age-dependent morphologic-functional changes of the equine cardiovascular system. In elderly humans, myocardial fibrosis formation and increasing stiffness of the myocardium have been described [17]. Loss of vascular elasticity increases cardiac afterload leading, in particular, to myocardial hypertrophy of the left ventricle [18]. These changes impair the impulse forming and conduction systems and result in decreasing contraction velocities during systole and delayed myocardial relaxation during diastole [17]. Reduced early diastolic filling pressures are compensated by increased late diastolic ventricular filling by active atrial contraction to keep the stroke volume constant [35].
It has been shown in the horse that beginning at an age of 15 years vascular wall thickness increases, while elastic fibers and smooth muscle mass decrease leading to an overall loss of elasticity. Consequently, increases in afterload and cardiac workload occur. Many conclusions on equine morphologic cardiac remodeling have been drawn from human medicine; for example, the loss of contraction by the replacement of cardiomyocytes with collagen fibers and connective tissue [23]. A reduction in the number of myocytes and accompanying hypertrophy of the surviving cells was observed in humans [23]. Nevertheless, cardiomyocytes of the left free wall were enlarged compared to those on the right side in horses of all age groups. An explanation for this lies in the five-times higher afterload of the left ventricle in comparison to the right ventricle; however, it is difficult to define the border between physiologic adaption and pathologic cardiac remodeling. Therefore, we used the modern examination technique of gray-scale speckle tracking (ST) in this study to achieve high sensitivity in identifying age-related changes in the equine myocardium.
In human medicine, gray-scale ST is a well-accepted diagnostic in examining heart disease [141012131519203234]. Ultrasonographic images are analyzed by this technique to evaluate different deformation parameters of different locations within the cardiac musculature. In particular, these measurements are of interest during the early diagnosis of cardiac disease before morphologic changes become easily visible. Gray-scale ST is used to evaluate the myocardial deformation strain and strain rate parameters and is based on the fact that a two-dimensional echocardiography (2DE) ultrasonogram consists of multiple shades of gray. The speckles appear due to interference of reflected ultrasonographic waves with nearby myocardial structures. Small areas within the myocardium have a specific gray-scale pattern, and there is software available that is capable of following these areas from image to image and calculating myocardial motion throughout the cardiac cycle [2]. Pattern recognition is based on the analysis of the ultrasonographic wave itself or on the demodulated signal as a brightness value [35].
In human medicine, strain and strain rate in stress echocardiography are used for the diagnosis of ischemia [14,16], for the evaluation of asynchronism [9,31], and for identifying myocardial function in cases of valve insufficiencies [9]. These myocardial deformation parameters, strain and strain rate, have been shown in humans to decrease with age [517].
Various studies have been published in equine medicine on different aspects and the clinical benefit of two-dimensional ST (2D-ST) in the horse. Most authors have used the right caudal short axis for the evaluation of radial strain and strain rate [212425]. In this echocardiographic view the displayed cross-section of the left ventricle is automatically divided into six myocardial segments. The maximum velocities and the amounts of myocardial motion/deformation can be calculated for those segments. In comparison to tissue Doppler imaging (TDI) most authors note a much lower reproducibility of 2D-ST data due to relatively high mean variation. Measurements of systolic parameters of the radial left ventricular free wall (LVFW) were found to be most reliable. In horses with cardiac valve insufficiencies, an increase of strain has been reported. In cases with increased heart dimensions, strain and systolic peak of strain rate in the interventricular septum (IVS) were reduced compared to those in healthy controls.
The correct interpretation of this very sensitive diagnostic imaging technique includes adequate assessment of sources of error and possible influencing factors. Therefore, this study focused on the physiologic aging process in the horse. Histopathologic evidence of cardiac remodeling with increasing age exists. Consequently, we evaluated the extent to which these changes can be detected using 2D-ST and if age-related changes influence the acquired data. Age-related structural changes of the heart influence myocardial deformation in humans; however, no reports on the influence of age on myocardial deformation have been published in veterinary medicine.
In this study, 57 horses used for pleasure riding in the region of Berlin, Germany were examined. Inclusion criteria were as follows: warmblood, age 3 to 30 years, no abnormal general clinical examination, electrocardiography (ECG), or echocardiography results. The horses were divided into five age-based groups (Group 1, 3–8 years [n = 14]; Group 2, 9–13 years [n = 7]; Group 3, 14–18 years [n = 10]; Group 4, 19–23 years [n = 10]; and Group 5, 24–30 years [n = 9]).
Sampling of horses affected by respiratory disease was not classified as animal experiments by the State Office of Health and Social Affairs Berlin (LaGeSo), sampling of control horses was approved (No. L0294/13). The owners gave permission to involve their horses in the study.
At the beginning of the echocardiographic examination, the electrodes were placed to obtain standard ECG at rest, for which the modified heart base-apex-lead was used.
The echocardiographic examination was performed by using a Vivid i (ver. 10.2.0.b.110; GE Healthcare, Norway) and a 3S-RS probe (frequency of 1.7/3.2 MHz). Analysis of images was performed by using the software Echopac (ver. 110.1.1; GE Healthcare). In all horses, the echocardiographic examination was performed in 2DE, M-mode, and color Doppler views (long and short axes). The 2D-ST analysis (radial strain) was performed offline and afterward imaging. The right caudal short axis (cross-section) was used for all measurements and required a frequency of at least 50 frames per second (fps) [25]; therefore, a narrow sector angle was used. The 2D-ST was performed for the LVFW and the IVS. For analysis, the gray-scale image had to be free of artefacts and of high image quality. Five cardiac cycles were recorded. These cine loops were analyzed by using the Echopac software described above. Six “regions of interest” (ROIs) were analyzed in the gray-scale images (Fig. 1). Visual control ensured correctness of ROI placement within the endocardium. Beginning from the IVS, the ROIs were placed clock-wise by marking the endocardial border. The option “process” allowed for the ROIs to be adapted automatically to the myocardium. A single ROI should be in the same heart wall throughout the entire cardiac cycle; therefore, the ROIs had to be corrected manually in many cases by focusing on the IVS (Fig. 2) and the LVFW (Fig. 3), which were of special interest in this study. This procedure was repeated three times for every horse and for three consecutive heart cycles.
Data analysis, including multiples regression analysis, was performed by using GNU R, freeware by Auckland University, Australia. To identify the transformation useful for variance stabilization, the optimal λ-coefficient was calculated by using the Box-Cox procedure. In many cases, transformation was not essential (λ ≈ 1), but in others log-transformation (λ ≈ 0) or reciprocal transformation (λ ≈ −1) was useful. The myocardial deformation parameters were defined as dependent variables, while age and other parameters (sex, height, weight, heart rate, and sports discipline) were deemed non-dependent variables. Further, possible interactions between non-dependent variables were tested for measurable variables. The variability of single measurements was expressed by calculating the coefficient of variation. A p value ≤ 0.05 was defined as the level of statistical significance. In addition, the coefficient of determination (R2) was calculated. For the statistical analysis of descriptive metrics, the study population was divided into five groups. The data are presented as mean ± SD values.
The hypothesis was that the age influences the myocardial deformation parameters of the equine heart and that these influences can be measured by using 2D-ST.
Of the 57 horses, aged 3 to 30 years, examined 33 were males and 24 females. The horses were selected to achieve homogeneous age diversity and comparable group sizes. Height varied from 155 to 180 cm and weight from 434 to 630 kg. All horses were examined at rest and had not exercised immediately before the examination. Heart rate at rest varied from 26 to 44 beats per minute. Concerning the sports discipline, there was a high negative correlation between age and exercise intensity; the older the horse, the less its regular exercise intensity tended to be. Therefore, sports discipline was excluded from the regression analysis.
Unremarkable general clinical examination results were an inclusion criterion for this study. Therefore, horses with a history of or presenting with an abnormal examination results were excluded. All 57 horses included in the statistical analysis had normal general clinical examination results.
All horses presented with normal mucous membranes, skin turgor, and arterial and venous pulse tests. Auscultation revealed a diastolic murmur on the aortic valve grade 1 to 2.
The standard ECG at rest showed a regular rhythm with regularly reoccurring P-QRS-T complexes in all horses.
The heart dimensions were within the normal limits reported for warmblood horses [30].
In 11 horses, aortic valve regurgitation was detected by using color Doppler imaging. To evaluate the clinical relevance and volume of the regurgitation, continuous wave-Doppler imaging was used. Aortic valve regurgitations were all < 2 m/sec and, therefore, classified as hemodynamically irrelevant. All horses with aortic valve regurgitation were ≥ 18 years old.
By using 2D-ST, correlations of age with strain and strain rate were evaluated in the IVS and the LVFW. Five of the 57 horses were excluded from 2D-ST due to poor image quality, thus 52 horses were included in the statistical analysis.
A significant increase in radial strain rate with age was observed for the LVFW during systole (p ≤ 0.05) and early diastolic relaxation (p ≤ 0.01; Table 1). No effect of age was detected during late diastolic contraction. In addition, age did not influence strain. Fig. 1 shows the absence of a correlation between age and strain for the LVFW and the IVS. A relatively high distribution of data resulted in no significant effect of age; age-strain analysis revealed a low R2 value.
A significant increase of strain rate with age was also observed for the IVS during systolic and late diastolic contraction (p ≤ 0.05). However, no significant effects of age on IVS were found for strain or early diastolic relaxation. In Table 2, mean and standard distribution values are presented for the different age groups of strain and strain rate in the IVS. Fig. 4 shows the strain and Fig. 5 the strain rate relationships with age for the LVFW and the IVS. The p and R2 are shown for each time point and each wave. The strain rates had generally low R2 values.
The coefficients of variation of the single measurements in the 2D-ST analysis were generally high (Table 3). In particular, the diastolic strain rate had high coefficients of variation, with up to 39.5% coefficients being obtained.
Possible further influencing factors on strain and strain rate including sex, weight, height, and heart rate were also evaluated. A weak effect of height on strain and strain rate was found for the A-wave in the IVS (p ≤ 0.05). Therefore, taller horses tended to have a higher myocardial deformation at this time point, but as the R2 value was low (0.15), this relationship was likely a coincidence.
Myocardial deformation parameters can be evaluated by using TDI, as well as 2D-ST, but there are differences between the techniques. From a clinical perspective, 2D-ST may be seen as an extension of echocardiography, as the acquired data describe heart function specifically. Independent of angle, global heart movement as well as passive movement of myocardial areas (tethering) are important differences when acquiring deformation parameters via TDI [23830]. Both TDI and 2D-ST have been used in equine cardiology to evaluate deformation parameters. There is a low reproducibility of data for color TDI due to the angle effect [27], and the feasibility of using 2D-ST is controversial [67]. Schwarzwald et al. [26] described radial and circumferential strain and strain rate as useful; in particular, systolic radial left ventricular values were relatively reliable. However, interobserver variability was high; therefore, the authors stressed the importance of intensive training, strict application guidelines, and clear definitions of gray-scale images.
To date, there are no reports in equine medicine focusing on the physiologic effect of age on the deformation parameters of the heart. Studies in humans have produced controversial results [2227]. Sun et al. [30] found no effect of age on strain and strain rate with the exception of late diastolic strain rate, for which an increase with age was observed. In that study, deformation parameters were evaluated by using TDI, and the authors suspected that low image quality masked a possible age effect. Kuznetsova et al. [16] also evaluated left ventricular strain and strain rate using TDI in healthy humans and reported an age-dependent decrease of longitudinal and radial strain and strain rate. Dalen et al. [5] studied strain and strain rate of the left ventricle in a large number (1266) of healthy subjects using TDI and 2D-ST. The acquired data showed decreases in end-systolic global strain and maximum systolic strain rate. However, diastolic deformation parameters were not studied. In addition, Dalen et al. [5] used their own adapted software for 2D-ST analysis, as commercial software had been criticized for accepting segments for analysis that did not have optimum tracking quality.
For the evaluation of a possible age effect, two myocardial segments were evaluated in this study by using the right ventricular short axis: IVS and LVFW. The acquired 2D-ST curves were similar to those reported in previous equine studies [2226]. Analysis of our data revealed increases of S- and E-waves with age in the LVFW. The late diastolic deformation rate was not influenced by age. Increases in S- and A-waves with age was also observed in the IVS but no influence on the E-wave. Strain values were found to be independent of age. On the basis of studies in human medicine, an age-dependent decrease in deformation parameters had been expected. The focus on transmural strain and strain rate might have been the reason for not detecting changes in these sensitive parameters. Magnetic resonance imaging-derived longitudinal and radial measurements have shown that strain increases in humans from 15% in the base to 19% in the apex of the heart [21]. In our study, the measurements were performed at cordal level, which might explain why the strain was lower than at a level closer to the apex. In a longitudinal direction, the number of patterns detectable in the myocardium is higher than that in a radial direction. Therefore, data acquired using a short axis is less robust than data from a longitudinal axis.
In addition, lateral resolution is important when tracking speckles. Resolution is influenced by the image rate: the higher the image rate, the lower the lateral resolution [2]. In the horse, the low heart rate at rest allows a relatively low image rate. A rate of 60 to 70 fps is recommended, which was fulfilled at a rate of 64.1 fps [33]. On the other hand, essential tissue depth can also influence lateral resolution: the greater the depth, the lower the lateral resolution and the poorer the tracking of speckles [2]. To address this and optimize lateral resolution, we chose the narrowest sector angle and the lowest tissue depth possible.
Additionally, the so-called “drift” has an important role that must not be neglected. A “kernel” is an area of defined size, which includes some speckles that are followed from image to image. Due to minimal changes in reflection intensity caused by variations in angle position, these kernels may not be identified correctly from image to image, leading to a reduced 2D-ST specificity [29]. During systole, the base of the ventricle moves toward the apex. This movement is another reason that the gray-scale pattern varies to a small extent between single images [2]. The high risk of artefacts as well as anatomic limitations in probe positioning lead to high coefficients of variation for 2D-ST and may mask the effects of age on deformation parameters. As strain increases toward the apex of the heart, a lower cross-section may show more significant differences. In human and small animal medicine, 2D-ST is mainly used for measurement of longitudinal strain and strain rate. This is impossible in the horse and leads to clinical limitations in the application of this technique. Therefore, in routine diagnostics of the horse, TDI is thought to have a higher value. However, TDI only allows for the acquisition of one-dimensional data, while the heart walls have three-dimensional movements throughout the cardiac cycle [27282934]. Further, it needs to be considered that deformation of the segment being evaluated is influenced by myocardial segments nearby, an effect called tethering [211]. Such tethering may mask observation of areas affected by cardiac disease by hindering the detection of missing movements when using TDI [193032]. Deformation parameters are not influenced by other segments; therefore, they may have a higher value in the evaluation of regional contractility.
Figures and Tables
Fig. 1
Right parasternal short axis beneath the mitral valve: automatic placement of region of interest segments.
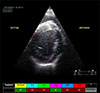
Fig. 2
Radial strain in the interventricular septum (purple) and the left ventricular free wall (yellow).

Fig. 3
Radial strain rate in the interventricular septum (purple) and the left ventricular free wall (yellow).
Fig. 4
Strain at different ages in the interventricular septum (IVS) and the left ventricular free wall (LVFW).

Fig. 5
Strain rate in the left ventricular free wall (LVFW) and the interventricular septum (IVS) at different ages. S, systolic maximum velocity; E, early diastolic filling; A, atrial release.

References
1. Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, Støylen A, Ihlen H, Lima JA, Smiseth OA, Slørdahl SA. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol. 2006; 47:789–793.

2. Artis NJ, Oxborough DL, Williams G, Pepper CB, Tan LB. Two-dimensional strain imaging: a new echocardiographic advance with research and clinical applications. Int J Cardiol. 2008; 123:240–248.

3. Castro PL, Greenberg NL, Drinko J, Garcia MJ, Thomas JD. Potential pitfalls of strain rate imaging: angle dependency. Biomed Sci Instrum. 2000; 36:197–202.
4. Cho GY, Chan J, Leano R, Strudwick M, Marwick TH. Comparison of two-dimensional speckle and tissue velocity based strain and validation with harmonic phase magnetic resonance imaging. Am J Cardiol. 2006; 97:1661–1666.

5. Dalen H, Thorstensen A, Aase SA, Ingul CB, Torp H, Vatten LJ, Stoylen A. Segmental and global longitudinal strain and strain rate based on echocardiography of 1266 healthy individuals: the HUNT study in Norway. Eur J Echocardiogr. 2010; 11:176–183.

6. Decloedt A, Verheyen T, Sys S, De Clercq D, van Loon G. Quantification of left ventricular longitudinal strain, strain rate, velocity, and displacement in healthy horses by 2-dimensional speckle tracking. J Vet Intern Med. 2011; 25:330–338.

7. Decloedt A, Verheyen T, Sys S, De Clercq D, van Loon G. Two-dimensional speckle tracking for quantification of left ventricular circumferential and radial wall motion in horses. Equine Vet J. 2013; 45:47–55.

8. D'hooge J, Heimdal A, Jamal F, Kukulski T, Bijnens B, Rademakers F, Hatle L, Suetens P, Sutherland GR. Regional strain and strain rate measurements by cardiac ultrasound: principles, implementation and limitations. Eur J Echocardiogr. 2000; 1:154–170.
9. Di Salvo G, Russo MG, Paladini D, Pacileo G, Felicetti M, Ricci C, Cardaropoli D, Palma M, Caso P, Calabro R. Quantification of regional left and right ventricular longitudinal function in 75 normal fetuses using ultrasound-based strain rate and strain imaging. Ultrasound Med Biol. 2005; 31:1159–1162.

10. Dohi K, Suffoletto M, Murali S, Bazaz R, Gorcsan J. Benefit of cardiac resynchronization therapy to a patient with a narrow QRS complex and ventricular dyssynchrony identified by tissue synchronization imaging. Eur J Echocardiogr. 2005; 6:455–460.

11. Heimdal A, Støylen A, Torp H, Skjaerpe T. Real-time strain rate imaging of the left ventricle by ultrasound. J Am Soc Echocardiogr. 1998; 11:1013–1019.

12. Ihaka R. R: past and future history. Comput Sci Stat. 1998; 392–396.
13. Jamal F, Strotmann J, Weidemann F, Kukulski T, D'hooge J, Bijnens B, Van de Werf F, De Scheerder I, Sutherland GR. Noninvasive quantification of the contractile reserve of stunned myocardium by ultrasonic strain rate and strain. Circulation. 2001; 104:1059–1065.

14. Kaluzynski K, Chen X, Emelianov SY, Skovoroda AR, O'Donnell M. Strain rate imaging using two-dimensional speckle tracking. IEEE Trans Ultrason Ferroelectr Freq Control. 2001; 48:1111–1123.

15. Kukulski T, Jamal F, Herbots L, D'hooge J, Bijnens B, Hatle L, De Scheerder I, Sutherland GR. Identification of acutely ischemic myocardium using ultrasonic strain measurements: a clinical study in patients undergoing coronary angioplasty. J Am Coll Cardiol. 2003; 41:810–819.

16. Kuznetsova T, Herbots L, Richart T, D'hooge J, Thijs L, Fagard RH, Herregods MC, Staessen JA. Left ventricular strain and strain rate in a general population. Eur Heart J. 2008; 29:2014–2023.

17. Lakatta EG. Cardiovascular aging research: the next horizons. J Am Geriatr Soc. 1999; 47:613–625.

18. Marcomichelakis J, Withers R, Newman GB, O'Brien K, Emanuel R. The relation of age to the thickness of the interventricular septum, the posterior left ventricular wall and their ratio. Int J Cardiol. 1983; 4:405–419.

19. Miyatake K, Yamagishi M, Tanaka N, Uematsu M, Yamazaki N, Mine Y, Sano A, Hirama M. New method for evaluating left ventricular wall motion by color-coded tissue Doppler imaging: in vitro and in vivo studies. J Am Coll Cardiol. 1995; 25:717–724.

20. Moore CC, Lugo-Olivieri CH, McVeigh ER, Zerhouni EA. Three-dimensional systolic strain patterns in the normal human left ventricle: characterization with tagged MR imaging. Radiology. 2000; 214:453–466.

21. Nagel D, Gehlen H. [The effectiveness of romifidine on myocardial function in horses with and without heart disease, evaluated with M-mode echocardiography and PW-tissue Doppler imaging]. Berl Munch Tierarztl Wochenschr. 2013; 126:436–443. German.
22. Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991; 68:1560–1568.

23. Sage AM. Cardiac disease in the geriatric horse. Vet Clin North Am Equine Pract. 2002; 18:575–589. viii

24. Schefer KD, Bitschnau C, Weishaupt MA, Schwarzwald CC. Quantitative analysis of stress echocardiograms in healthy horses with 2-dimensional (2D) echocardiography, anatomical M-mode, tissue Doppler imaging, and 2D speckle tracking. J Vet Intern Med. 2010; 24:918–931.

25. Schwarzwald CC, Schober KE, Berli AS, Bonagura JD. Left ventricular radial and circumferential wall motion analysis in horses using strain, strain rate, and displacement by 2D speckle tracking. J Vet Intern Med. 2009; 23:890–900.

26. Schwarzwald CC, Schober KE, Bonagura JD. Methods and reliability of tissue Doppler imaging for assessment of left ventricular radial wall motion in horses. J Vet Intern Med. 2009; 23:643–652.

27. Sengupta PP, Krishnamoorthy VK, Korinek J, Narula J, Vannan MA, Lester SJ, Tajik JA, Seward JB, Khandheria BK, Belohlavek M. Left ventricular form and function revisited: applied translational science to cardiovascular ultrasound imaging. J Am Soc Echocardiogr. 2007; 20:539–551.

28. Stadler P, Robine F. [Die Kardiometrie beim gesunden Warmblutpferd mit Hilfe der Schnittbildechographie im B-Mode]. Pferdeheilkunde. 1996; 12:35–43. German.
29. Stoylen A, Heimdal A, Bjornstad K, Torp HG, Skjaerpe T. Strain rate imaging by ultrasound in the diagnosis of regional dysfunction of the left ventricle. Echocardiography. 1999; 16:321–329.

30. Sun JP, Chinchoy E, Donal E, Popović ZB, Perlic G, Asher CR, Greenberg NL, Grimm RA, Wilkoff BL, Thomas JD. Evaluation of ventricular synchrony using novel Doppler echocardiographic indices in patients with heart failure receiving cardiac resynchronization therapy. J Am Soc Echocardiogr. 2004; 17:845–850.

31. Sun JP, Popović ZB, Greenberg NL, Xu XF, Asher CR, Stewart WJ, Thomas JD. Noninvasive quantification of regional myocardial function using Doppler-derived velocity, displacement, strain rate, and strain in healthy volunteers: effects of aging. J Am Soc Echocardiogr. 2004; 17:132–138.

32. Teske AJ, De Boeck BW, Melman PG, Sieswerda GT, Doevendans PA, Cramer MJ. Echocardiographic quantification of myocardial function using tissue deformation imaging, a guide to image acquisition and analysis using tissue Doppler and speckle tracking. Cardiovasc Ultrasound. 2007; 5:27.

33. Thomas JD, Popović ZB. Assessment of left ventricular function by cardiac ultrasound. J Am Coll Cardiol. 2006; 48:2012–2025.

34. Voigt I, Mansi T, Ionasec RI, Mengue EA, Houle H, Georgescu B, Hornegger J, Comaniciu D. Robust physically-constrained modeling of the mitral valve and subvalvular apparatus. In : 14th International Conference on Medical Image Computing & Computer Assisted Intervention: MICCAI 2011; 18–22 Sep 2011; Toronto, Canada.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download