Abstract
This study investigated the correlation between oxidative stress status and key canine sperm parameters and the effect of addition of a superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) combination in egg yolk tris-citrate glucose (EYT-G) extender on semen during 10 days of storage at 4℃. Ten Boxer dogs were divided into two groups, fertile (F) and hypofertile (H), depending on pregnancy and live birth rate status in the previous year. Semen evaluation was performed on the day of collection (D0) and after 5 (D5) and 10 (D10) days of cooled storage. Sperm motility, kinetic parameters, and DNA integrity were assessed. A correlation between oxidative status and key semen parameters in both F and H groups was observed. Total and progressive motilities were significantly higher in the treated (SOD, CAT, and GPx addition) versus control groups at D10 in both F and H groups, and at D5 in the H group. DNA integrity was significantly higher in both treated groups (H and F) at D5 and D10. In conclusion, the addition of SOD, CAT, and GPx in the extender allows preservation of semen quality for up to 10 days of storage at 4℃ in both fertile and hypofertile dogs.
Artificial insemination (AI) is practiced frequently in domestic dog breeding for genetic improvement of breeds, to satisfy the demands of private owners, and for the preservation of endangered canids. AI has been accepted worldwide, supporting ex situ conservation programs for endangered canine species [21]. Canine AI can be done with fresh, chilled, or frozen semen. Currently, the use of cooled semen is increasing in canines as it can be stored for relatively long periods resolving problems associated with AI timing and shipping semen over long distances. Shipped semen can be utilized for trans-vaginal and trans-cervical insemination, and it requires inexpensive equipment and provides improved pregnancy rates [33]. AI techniques in breeding programs involving chilled canine semen require reliable long-term storage conditions to preserve sperm quality. In order to preserve spermatozoa during storage, their metabolic activities need to be reduced by using an appropriate medium and storage temperature. Hori et al. [18] demonstrated that semen qualities can be maintained for up to 48 h when canine semen samples are extended with egg yolk tris-citrate fructose (EYT-F) or glucose (EYT-G) and stored at a temperature in the range of 4℃ to 12℃. Several extenders to preserve fertilizing capacity of canine semen have been successfully tested, but further studies are indispensable to improve the quality of cooled stored semen [922].
There is little available information about the molecular mechanisms leading to death of spermatozoa during refrigeration, and apoptosis has recently been shown to be involved [2439]. Also, it has been reported that cryopreservation results in death by apoptosis of equine, bovine, and human spermatozoa [28313236].
Sperm survival during refrigeration depends on oxygen consumption and metabolism, which increase the amount of reactive oxygen species (ROS) [3]. Although low ROS amounts are needed by spermatozoa to acquire fertilizing capabilities, excessive ROS production can damage sperm motility, sperm-oocyte fusion, and impair fertilization [5]. Due to the high sensitivity of spermatozoa to oxidative stress, ROS concentration is controlled by endogenous antioxidants, thereby avoiding ROS overload and preserving semen quality [162937]. These antioxidant systems consist of enzymatic antioxidants, such as SOD, CAT, and GPx, and non-enzymatic antioxidants (vitamin A, C, E, and uric acid). A study on dogs confirmed the presence of endogenous antioxidants in the seminal plasma of pre-spermatic, spermatic, and post-spermatic fractions, with SOD representing the major enzymatic antioxidant in all dog ejaculate fractions, whereas GPx activity was present in the sperm-rich and post-spermatic fractions, and CAT activity was deficient [38]. Another study [23], however, demonstrated CAT activity in dog ejaculates and showed that the addition of SOD and CAT in the dilution extender of canine semen improved sperm quality.
The aim of this study was to investigate the effect of addition of SOD, CAT, and GPx in EYT-G extender on the survival of spermatozoa of dogs with different oxidative status. Sperm motility, kinetic parameters, and DNA integrity were evaluated during 10 days of storage at 4℃.
Animals In order to select 10 dogs to place into each of two study groups, fertile (F) and hypofertile (H), a pilot study was conducted on 20 male Boxer dogs. A complete history, physical examination, and semen evaluation were performed on each dog. The age range of the selected dogs was 2 to 7 years (mean ± SE, 4.5 ± 1.2 years). The pregnancy and live birth rates of the preceding year after natural breeding and the fertility-related semen parameters of undiluted fresh semen such as concentration, motility, and morphology of spermatozoa were used to distinguish hypofertile and fertile dogs. Among the 20 dogs, the 5 with higher pregnancy and live birth rates (at least 1 live birth with at least 4 live and viable puppies) and with fertility-related parameters above average were included in the F group. The 5 dogs with no record of a live birth in the preceding year and with fertility-related parameters below average were included in the H group.
The dogs were housed in a domestic situation in Naples, Italy. Commercial dog food was given once daily, and water was given thrice daily (morning, afternoon, and evening). The owners of the dogs included in the study provided written consent, and the study was conducted in conformity with the animal study guidelines of the Naples Veterinary Medicine and Animal Production Department (D.lgs.26-04/03/2014).
In the first step of our study, we evaluated the quality of the dogs' ejaculates and their systemic oxidative state. To this aim, one ejaculate and one blood samples were harvested from each dog, for a total of 10 semen samples and 10 blood samples (from 5 fertile and 5 hypofertile dogs).
In the second step, the effect of enzymatic antioxidant supplementation on the cooling resistance of semen collected from fertile and hypofertile dogs was evaluated. To this aim, ejaculate from each dog in the F and H groups was divided into 2 aliquots (hypofertile and fertile controls [HC and FC]; hypofertile and fertile experimental [HE and FE]) and prepared as described below for storage at 4℃ with or without antioxidant supplementation in the extender. Diluted and refrigerated samples were stored for 10 days. For each aliquot, semen quality (motility and kinetic parameters) and DNA fragmentation were evaluated at day 0, day 5, and day 10 of storage.
Blood samples were collected via cephalic vein, and sera collected after centrifugation were frozen, stored at −20℃, and analyzed within one month from collection.
Pro-oxidant and antioxidant status of dogs was evaluated on serum by using the d-ROMs test and the OXY-adsorbent test. All test kits were purchased from Diacron International (Italy) [10].
Pro-oxidative status was evaluated by applying the d-ROMs test, a photometric test that allows the determination of reactive oxygen metabolite (ROM) concentration, in particular, hydroperoxide concentration. The d-ROMs test measures the oxidant ability of a serum sample (10 µL) toward a particular substance (modified aromatic amine) that is used as an indicator (chromogen, N,N-diethylparaphenylendiamin). The intensity of the developed color, photometrically quantified at 505 nm, is directly proportional to the concentration of the ROMs, according to the Lambert-Beer's law and is expressed as Carratelli units (1 CARR U = 0.08 mg hydrogen peroxide/dL).
Antioxidant capacity (OXY) was measured as the ability of plasma to counteract the massive oxidation induced by a solution of hypochlorous acid (HClO), a powerful and physiological oxidant able to mimic situations that occur in vivo. Unreactive HClO radicals further react with the chromogen solution of N,N-diethylparaphenylendiamin and form a colored complex, which is measured at 505 nm. The results were expressed as µmol HClO/L.
The degree of oxidative stress was expressed by the oxidative stress index (OSi) [2], which is calculated as a ratio between pro-oxidants and antioxidants by using the following formula:
Dog semen was obtained manually by masturbation at room temperature (25℃) [318] after the males had been accustomed to their housing and to the individual collecting the semen.
The semen was collected into sterile plastic test tubes (Falcon tube; Minitübe, Germany) and stored in a water bath at 37℃ for 1 h. The pH and volume of each ejaculate were measured. Semen samples were divided into three fractions, and the second fraction, called the sperm-rich fraction, was used in this study. This fraction was initially evaluated macroscopically and then examined in detail under a microscope. Sperm concentration was determined by using a hemocytometer (Burker chamber) and sperm motility was examined on glass slides on a heated stage at 37℃ under a Nikon TE 2000 inverted microscope (Nikon, Italy) connected to a Basler Vision Technology A312 FC camera (Basler, Germany) under phase-contrast at 400× [18]. Sperm viability and morphology were assessed by applying an eosin viability stain (Europath, Italy). Abnormal morphology was determined by examining at least 100 spermatozoa at 400× magnification under phase-contrast microscopy. Total and progressive motilities were evaluated by an expert observer using a phase-contrast microscope with a heated stage at 37℃ at 100× magnification on ten randomly selected fields for each sample.
Storage methods were performed as previously reported [40]. Samples were centrifuged at 650 × g for 5 min to remove seminal plasma and, then, divided into two aliquots. The sperm concentration of each aliquot was adjusted to 100 × 106 sperm/mL in EYT-G extenders. The EYT-G extender was composed by tris(hydroxymethyl)-aminomethane (2.422 g), citrate acid monohydrate (1.360 g), glucose (1 g), gentamycin (100 mg), egg yolk (20 mL), and distilled water (80 mL), and, if needed, pH was adjusted to 7.0 with hydrogen chloride. Each experimental aliquot was supplemented with 15 IU/mL of GPx, 15 IU/mL of CAT, and 15 IU/mL of SOD. The total amount of antioxidants added to EYT-G was based on previous studies in human and horses [113]. The experimental (FE and HE) and control (FC and HC) aliquots were placed in a syringe without air and stored in the fridge at 4℃ for 10 days. Semen quality tests were performed at the time of semen collection (D0), and at 5 (D5) and 10 (D10) days of post-cooling storage at 4℃. To prevent a rapid temperature drop, samples were stored in a beaker containing 500 mL of water at room temperature. In addition, to reduce the effects of ambient temperature, the experiments were conducted in a room with a controlled temperature of 20℃.
Semen was examined before and after cooling at different times, and total and progressive motilities (%), kinetic parameters, and DNA integrity (%) were evaluated as described below.
Moreover, semen analysis was performed by using the Sperm Class Analyzer CASA system (SCA; Microptic, Spain). Briefly, a 10-µL aliquot of semen sample was placed in a Makler chamber on a heated stage at 37℃, after pre-incubation at 37℃ for 10 min. At least 200 spermatozoa were counted to evaluate the percentages of motile (MOT) and progressively motile (PMOT) spermatozoa, as well as rapid (RAP), medium (MED), slow (SLOW), and static (STATIC) spermatozoa, and sperm kinetic parameters, i.e., curvilinear velocity (VCL), straight line velocity (VSL), average path velocity (VAP), linearity (LIN = VSL/VCL), and straightness (STR = VSL/VAP). The SCA settings were adjusted according to the manufacturer's instructions. All manual and SCA measurements were performed by trained and licensed medical technologists. The SCA results were used when the difference between manual and SCA values was less than 20%. In cases where the difference was greater than 20%, the manual results were reported [17].
As shown in Fig. 1, DNA fragmentation was evaluated by using the APO-BrdU TUNEL Assay Kit (Molecular Probes, A35125; Invitrogen, USA) as described by Boni et al. [8]. Sperm cells were prepared and fixed by using paraformaldehyde as required by the APO-BrdU terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate (dUTP)-nick end-labeling (APO-BrdU TUNEL) assay.
Samples were centrifuged at 1,100 × g for 10 min and the sperm pellets were re-suspended to a final concentration of 1 × 106 cells in 0.5 mL of phosphate-buffered saline (PBS). Then, to each sperm suspension was added 5 mL of 1% (w/v) paraformaldehyde in PBS, after which, it was placed on ice for 15 min, centrifuged twice for 5 min at 600 × g. The obtained pellet was re-suspended in 0.5 mL of PBS and permeabilized through the addition of 5 mL of ice-cold ethanol 70% (v/v). Fixed samples were stored at −20℃ and stained within 1 week.
TUNEL-labeled spermatozoa were analyzed under a fluorescence microscope (Nikon Eclipse 90i; Nikon) equipped with a mercury lamp (100 W). Final detection of BrdU incorporation at DNA break sites was achieved through an Alexa Fluor 488 dye-labeled anti-BrdU antibody (Invitrogen, Italy). The Alexa Fluor 488 dye has excitation and emission maxima of 495/519 nm. Propidium iodide was excited at 488 nm and detected via a 560 long-pass filter, according to previously published methods [15]. For each sample, 10 slides were prepared and independently analyzed by two expert cytologists counting at least 100 spermatozoa for each slide at 400× final magnification.
Statistical analysis was carried out by using non-parametric statistic tests in statistical analysis software (IBM SPSS Statistics ver. 22.0; IBM, USA). Correlations between semen parameters of fresh diluted semen and OSi were determined by using Spearman's test. Interpretation of obtained Spearman's correlation coefficients was based on: 0 ≤ r < 0.6: low correlation; 0.6 ≤ r ≤ 1: high correlation.
The effect of storage time on semen parameters was tested by applying the Friedman ANOVA test in both control and experimental groups. Further analysis was carried out to determine whether the quality parameters of semen in the treated groups (FE and HE) were significantly different from those of the control group (FC and HC, respectively) by using the Wilcoxon signed rank test. Significance was set at p ≤ 0.05 and p ≤ 0.01.
Fertility-related semen parameters such as volume and sperm concentration, viability, total and progressive motilities, and morphology of spermatozoa in fresh undiluted semen of each of the ten dogs are reported in Table 1.
The pro-oxidant, antioxidant, and oxidative stress status of both groups are presented in Table 2, as d-ROMs, OXY adsorbent, and OSi results, respectively. There were statistically significant differences between groups with respect to d-ROMs and OSi.
The correlations between each seminal parameter and OSi were evaluated separately in the F and H groups. In the H group, there was a significant positive correlation between OSi and SLOW (r = 1.000), as well as OSi and STATIC (r = 1.000), and a significant negative correlation between OSi and MOT (r = −1.000) and OSi and RAP (r = −1.000). In the F group, there was a significant positive correlation between OSi and SLOW (r = 0.800) and a significant negative correlation between OSi and PMOT (r = −0.811). No significant correlation was detected between OSi and DNA fragmentation in either the F or H groups.
Fresh semen quality parameters of the F and H group dogs are presented in Fig. 2. The comparison between F and H groups showed significant differences (p < 0.05) for the following parameters: MOT, PMOT, RAP, MED, SLOW, STATIC, OSi, and DNA fragmentation. OSi was higher in the H group than in the F group. The data also showed a higher percentage of DNA fragmentation in spermatozoa of the H group than those of the F group (p < 0.05).
As expected, total and progressive motilities and DNA integrity were significantly (p < 0.01) reduced during cooled storage in each group, both in the control and experimental groups of fertile and hypofertile dogs. Significant changes associated with the storage time were found in sperm velocity parameters (RAP, MED, SLOW, and STATIC) and in sperm motion characteristics (VCL, VAP, and VSL), as shown in Tables 3 and 4, respectively. Straightness (STR) and linearity (LIN) were unaffected by time in fertile dogs in both the treated and control groups. However, straightness (STR) and linearity (LIN) of spermatozoa from hypofertile dogs showed a decrease with time; that decrease was greater in the HC group than in the HE group, especially at D5 of cooled storage. Furthermore, total and progressive motilities were significantly higher in the treated (FE and HE) groups than in the control (FC and HC) groups at D10, as well as at D5 in hypofertile dogs (HE group; panels A and B in Fig. 3). DNA fragmentation was significantly lower in treated (FE and HE) groups than in control (FC and HC) groups at D5 and D10 of cooled storage (p < 0.05; panel C in Fig. 3).
A fine balance between ROS production and an antioxidant system's ability to scavenge ROS is critical for normal cellular functions [12]. On this basis, a previous study in canines suggested the presence of correlations among individual oxidative status, endogenous antioxidant concentration in semen, and quality of semen that could influence the ability of sperm to withstand cryoinjury [30].
The influence of systemic and seminal oxidative status on semen quality parameters has been reported in humans [14] but not in dogs. As expected, in this study, differences in systemic oxidative status were evident between hypofertile and fertile dogs. Furthermore, for the first time, our results correlated systemic oxidative stress, measured by OSi, with semen quality in a canine species.
Although pregnancy rates remain the ultimate endpoint to evaluate the fertilizing capacity of canine semen, sperm motility and DNA integrity are commonly used to assess the quality of fresh and cooled semen. Sperm DNA damage is higher in infertile men than in fertile men, and such damage has been correlated with poor reproductive outcomes. Currently, in human assisted reproduction, sperm DNA damage, expressed as a DNA fragmentation index, can distinguish fertile and infertile men in clinical practice [14]. Our results show a similar difference in DNA integrity between hypofertile and fertile dogs.
Although the percentage of DNA fragmented spermatozoa in fresh semen was not correlated to OSi in either hypofertile and fertile dogs, we did obtain indirect evidence of a correlation between DNA integrity and oxidative stress during cooled storage, as the addition of antioxidants reduced the amount of DNA fragmentation. In contrast to our results, a previous study in canines suggested that the addition of vitamins C and E did not prevent DNA damage [26].
In the present study, the TUNEL assay was chosen to directly estimate DNA breakage, as it has been previously demonstrated to be a valid test in canines [20]. Herein, the addition of a combination of endogenous antioxidants to canine semen of subjects with different oxidative status was tested for the first time to improve semen preservation during cool storage. Several studies have reported that the addition of antioxidants to semen can improve the quality of preserved sperm [6719]. Although in human and horses the positive effects of single endogenous antioxidant supplementation have been tested [113], currently, there is little information available on the synergistic effects of different antioxidant combinations on the quality of canine semen stored under chilled conditions. Beneficial effects of a combination of antioxidants, including CAT, SOD, and GPx, on the quality of cryopreserved semen in rainbow trout (Oncorhynchus mykiss) have been reported [25]. Endogenous antioxidants differ among species [27], and SOD, CAT, and GPx activities have been demonstrated in canine semen [38]. In addition, a previous study [11] showed that cold storage compromised the SOD activity in the dog sperm cells; moreover, GPx appeared to work efficiently in conjunction with SOD to scavenge the ROS in chilled dog semen. Thus, it has been suggested that the addition of a GPx and SOD combination to a dog semen can protect sperm viability and DNA integrity [11]. Possibly, the synergistic effect of these antioxidants can preserve semen during chilled storage by lowering the concentration of ROS.
Herein, sperm motility and DNA integrity after 5 and 10 days of cooled storage were preserved by the addition of low concentrations of SOD, CAT, and GPx to the extender. Several studies have assessed the motility of chilled canine semen within 5 days of collection [183435]. Apoptosis is a major cause of sperm damage during cryopreservation [3]. Although the causes of such DNA damage have not been fully elucidated, several lines of evidence suggest that oxidative stress has a key role in the underlying etiology. Spermatozoa are particularly vulnerable to oxidative stress since they generate ROS and are rich in targets for oxidative attack. Furthermore, as spermatozoa are transcriptionally inactive and have little cytoplasm, they are deficient in both antioxidants and DNA-repair systems [4]. Compared to previous studies, the addition of low concentrations of SOD, CAT, and GPx to the dilution medium had allowed cooled storage of semen for 10 days with preserved sperm motility and DNA integrity. The prolonged preservation of cooled semen allows longer distance semen shipments and more precise timing of AI, thereby, further increasing fertility rates. Moreover, antioxidant addition might also further benefit spermatozoa within the female reproductive tract. It would be desirable to validate the results of such in vitro study through the assessment of the fertilizing ability of chilled semen supplemented with these antioxidants in vivo. To this end, it would be necessary to objectivize various parameters among female factors, male factors (e.g., individual and F and H groups), ovulation monitoring, and insemination techniques.
Taken together, the data obtained in this study demonstrate for the first time a correlation between oxidative stress and semen quality parameters in a canine species. Moreover, we have shown that supplementation of extenders with CAT, SOD, and GPx can preserve sperm motility and DNA integrity thereby improving cooled storage, especially in dogs with poor semen quality.
Figures and Tables
Fig. 1
Representative image of canine spermatozoa stained with an APO-BrdU TUNEL Assay Kit with anti-brdU Alexa Fluor 488 and using fluorescence microscopy at 400× magnification. Propidium iodide stains the nucleus of all sperm cells red and anti-brdU Alexa Fluor 488 stains spermatozoa with fragmented DNA in yellow. TUNEL-negative (red) and TUNEL-positive (yellow).
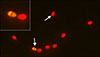
Fig. 2
Results of analysis of fresh diluted semen parameters in fertile and hypofertile groups: percentages of oxidative stress index (OSi), DNA integrity, total and progressive motilities (A); velocity parameters (B), and motion variables (C). VAP, average path velocity; VSL, straight line velocity; VCL, curvilinear velocity; STR, straightness; LIN, linearity. Significant differences between groups are denoted by *p < 0.05.
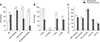
Fig. 3
Results of assessment of percentages of total (A) and progressive (B) motilities and DNA fragmentation (C) of diluted semen after 5 and 10 days of cooled storage in control and experimental groups of hypofertile (H) and fertile (F) dogs. Significant differences (*p < 0.05) between control and experiment groups.
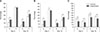
Table 3
Sperm velocity parameter results: measured by Sperm Class Analyzer CASA system within 5 and 10 days of cooled storage in control and experimental groups of both hypofertile and fertile dogs

Table 4
Sperm motion characteristic parameters: measured by using the Sperm Class Analyzer CASA system at 5 and 10 days of cooled storage in control and experimental groups of hypofertile and fertile dogs

Data are presented as mean ± SD. a–bStatistical difference (p < 0.05) between days of storage of each sperm motion characteristic parameter. VCL, curvilinear velocity; VAP, average path velocity; VSL, straight line velocity; STR, straightness (VSL/VAP); LIN, linearity (VSL/VCL). *Statistical difference (p < 0.05) between control and experiment groups.
Acknowledgments
This study was supported by Departmental Funds of Veterinary Medicine and Animal Productions, University of Naples Federico II, Italy.
References
1. Aboua YG, du Plessis SS, Reichgelt P, Brooks N. The in vitro effects of superoxide, some commercially available antioxidants and red palm oil on sperm motility. Asian J Androl. 2009; 11:695–702.

2. Abuelo A, Hernández J, Benedito JL, Castillo C. Oxidative stress index (OSi) as a new tool to assess redox status in dairy cattle during the transition period. Animal. 2013; 7:1374–1378.

3. Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003; 79:829–843.

4. Aitken J, Fisher H. Reactive oxygen species generation and human spermatozoa: the balance of benefit and risk. BioEssays. 1994; 16:259–267.

5. Aitken RJ, Clarkson JS, Fishel S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod. 1989; 41:183–197.

6. Aurich JE, Schönherr U, Hoppe H, Aurich C. Effects of antioxidants on motility and membrane integrity of chilled-stored stallion semen. Theriogenology. 1997; 48:185–192.

7. Beconi MT, Francia CR, Mora NG, Affranchino MA. Effect of natural antioxidants on frozen bovine semen preservation. Theriogenology. 1993; 40:841–851.

8. Boni R, Cocchia N, Silvestre F, Tortora G, Lorizio R, Tosti E. Juvenile and adult immature and in vitro matured ovine oocytes evaluated in relation to membrane electrical properties, calcium stores, IP3 sensitivity and apoptosis occurrence in cumulus cells. Mol Reprod Dev. 2008; 75:1752–1760.

9. Bouchard GF, Morris JK, Sikes JD, Youngquist RS. Effect of storage temperature, cooling rates and two different semen extenders on canine spermatozoal motility. Theriogenology. 1990; 34:147–157.

10. Cesarone MR, Belcaro G, Carratelli M, Cornelli U. A simple test to monitor oxidative stress. Int Angiol. 1999; 18:127–130.
11. Chatdarong K, Chaivechakarn A, Thuwanut P, Ponglowhapan S. Effects of cold storage prior to freezing on superoxide dismutase, glutathione peroxidase activities, level of total reactive oxygen species and sperm quality in dogs. Reprod Domest Anim. 2012; 47:Suppl 6. 274–277.

12. Chen SJ, Allam JP, Duan YG, Haidl G. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch Gynecol Obstet. 2013; 288:191–199.

13. Cocchia N, Pasolini MP, Mancini R, Petrazzuolo O, Cristofaro I, Rosapane I, Sica A, Tortora G, Lorizio R, Paraggio G, Mancini A. Effect of sod (superoxide dismutase) protein supplementation in semen extenders on motility, viability, acrosome status and ERK (extracellular signal-regulated kinase) protein phosphorylation of chilled stallion spermatozoa. Theriogenology. 2011; 75:1201–1210.

14. Cocuzza M, Sikka SC, Athayde KS, Agarwal A. Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: an evidence based analysis. Int Braz J Urol. 2007; 33:603–621.

15. De Iuliis GN, Thomson LK, Mitchell LA, Finnie JM, Koppers AJ, Hedges A, Nixon B, Aitken RJ. DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-2′-deoxyguanosine, a marker of oxidative stress. Biol Reprod. 2009; 81:517–524.

16. Griveau JF, Le Lannou D. Reactive oxygen species and human spermatozoa: physiology and pathology. Int J Androl. 1997; 20:61–69.

17. Hirano Y, Shibahara H, Obara H, Suzuki T, Takamizawa S, Yamaguchi C, Tsunoda H, Sato I. Relationships between sperm motility characteristics assessed by the computer-aided sperm analysis (CASA) and fertilization rates in vitro. J Assist Reprod Genet. 2001; 18:213–218.
18. Hori T, Ichikawa M, Kawakami E, Tsutsui T. Artificial insemination of frozen epididymal sperm in beagle dogs. J Vet Med Sci. 2004; 66:37–41.

19. Hu JH, Tian WQ, Zhao XL, Zan LS, Wang H, Li QW, Xin YP. The cryoprotective effects of ascorbic acid supplementation on bovine semen quality. Anim Reprod Sci. 2010; 121:72–77.

20. Jarosz Ł, Grądzki Z, Kalinowski M, Laskowska E. Quality of fresh and chilled-stored raccoon dog semen and its impact on artificial insemination efficiency. BMC Vet Res. 2016; 12:224.

21. Jewgenow K, Songsasen N. Reproduction and advances in reproductive studies in carnivores. In : Holt WV, Brown JL, Comizzoli P, editors. Reproductive Sciences in Animal Conservation. New York: Springer;2014. p. 205–239.
22. Kasimanickam VR, Kasimanickam RK, Memon MA, Rogers HA. Effect of extenders on sperm mitochondrial membrane, plasma membrane and sperm kinetics during liquid storage of canine semen at 5℃. Anim Reprod Sci. 2012; 136:139–145.

23. Kawakami E, Takemura A, Sakuma M, Takano M, Hirano T, Hori T, Tsutsui T. Superoxide dismutase and catalase activities in the seminal plasma of normozoospermic and asthenozoospermic Beagles. J Vet Med Sci. 2007; 69:133–136.

24. Kumi-Diaka J, Badtram G. Effect of storage on sperm membrane integrity and other functional characteristics of canine spermatozoa: in vitro bioassay for canine semen. Theriogenology. 1994; 41:1355–1366.

25. Kutluyer F, Kayim M, Öğretmen F, Büyükleblebici S, Tuncer PB. Cryopreservation of rainbow trout Oncorhynchus mykiss spermatozoa: effects of extender supplemented with different antioxidants on sperm motility, velocity and fertility. Cryobiology. 2014; 69:462–466.

26. Lopes-Santiago BV, Monteiro GA, Bittencourt R, Arduino F, Ovidio PP, Jordão-Junior AA, Araùjo JP Jr, Lopes MD. Evaluation of sperm DNA peroxidation in fertile and subfertile dogs. Reprod Domest Anim. 2012; 47:Suppl 6. 208–209.

27. Luberda Z. [Present conception regarding the effect on reactive oxygen species on functions of mammalian spermatozoa]. Postępy Biologii Komórki. 2001; 28:309–316. Polish.
28. Martin G, Sabido O, Durand P, Levy R. Cryopreservation induces an apoptosis-like mechanism in bull sperm. Biol Reprod. 2004; 71:28–37.

29. Michael AJ, Alexopoulos C, Pontiki EA, Hadjipavlou-Litina DJ, Saratsis P, Ververidis HN, Boscos CM. Quality and reactive oxygen species of extended canine semen after vitamin C supplementation. Theriogenology. 2008; 70:827–835.

30. Neagu VR, García BM, Rodríguez AM, Ferrusola CO, Bolaños JM, Fernández LG, Tapia JA, Peña FJ. Determination of glutation peroxidase and superoxide dismutase activities in canine seminal plasma and its relation with sperm quality and lipid peroxidation post thaw. Theriogenology. 2011; 75:10–16.

31. Ortega-Ferrusola C, Sotillo-Galán Y, Varela-Fernández E, Gallardo-Bolaños JM, Muriel A, González-Fernández L, Tapia JA, Peña FJ. Detection of “apoptosis-like” changes during the cryopreservation process in equine sperm. J Androl. 2008; 29:213–221.

32. Perrin A, Basinko A, Douet-Guilbert N, Gueganic N, Le Bris MJ, Amice V, De Braekeleer M, Morel F. Aneuploidy and DNA fragmentation in sperm of carriers of a constitutional chromosomal abnormality. Cytogenet Genome Res. 2011; 133:100–106.

33. Ponglowhapan S, Essén-Gustavsson B, Linde Forsberg C. Influence of glucose and fructose in the extender during long-term storage of chilled canine semen. Theriogenology. 2004; 62:1498–1517.

34. Province CA, Amann RP, Pickett BW, Squires EL. Extenders for preservation of canine and equine spermatozoa at 5℃. Theriogenology. 1984; 22:409–415.

35. Rota A, Ström B, Linde-Forsberg C. Effects of seminal plasma and three extenders on canine semen stored at 4℃. Theriogenology. 1995; 44:885–900.

36. Said TM, Gaglani A, Agarwal A. Implication of apoptosis in sperm cryoinjury. Reprod Biomed Online. 2010; 21:456–462.

37. Storey BT. Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Mol Hum Reprod. 1997; 3:203–213.

38. Strzezek R, Koziorowska-Gilun M, Kowalówka M, Strzezek J. Characteristics of antioxidant system in dog semen. Pol J Vet Sci. 2009; 12:55–60.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download