Abstract
In this study, we attempted to establish a culture system for in vitro spermatogenesis from spermatogonial stem cells (SSCs) of Bama mini-pig. Dissociated testicular cells from 1-month-old pigs were co-cultured to mimic in vivo spermatogenesis. The testicular cells were seeded in minimum essential medium alpha (α-MEM) supplemented with Knockout serum replacement (KSR). Three-dimensional colonies formed after 10 days of culture. The colonies showed positive staining for SSC-associated markers such as UCHL1, PLZF, THY1, OCT4, Dolichos biflorus agglutinin, and alkaline phosphatase. Induction of SSCs was performed in α-MEM + KSR supplemented with retinoic acid, bone morphogenetic protein 4, activin A, follicle-stimulating hormone, or testosterone. The results showed that STRA8, DMC1, PRM1, and TNP1 were upregulated significantly in the colonies after induction compared to that in testis from 1-month-old pigs, while expression levels of those genes were significantly low compared to those in 2-month-old testis. However, upregulation of ACROSIN was not significant. Replacement of α-MEM and KSR with Iscove's modified Dulbecco's medium and fetal bovine serum did not upregulate expression of these genes significantly. These results indicate that SSCs of Bama mini-pig could undergo differentiation and develop to a post-meiotic stage in α-MEM supplemented with KSR and induction factors.
Cultivation of spermatogonial stem cells (SSCs) and induction of spermatogenesis in vitro is a potential strategy for the generation of transgenic animals and the treatment of male infertility. In addition, induction of SSCs in vitro provides a strategy for use when exploring the factors controlling SSC differentiation. The potential usage value has provoked a number of researchers to focus on in vitro generation of sperm from male germline stem cells.
Initially, it was reported that spermatocytes and spermatids were derived from an immortalized cell line derived between spermatogonia B cells and primary spermatocytes in cultures supplemented with stem cell factors [9]. Subsequent studies showed that rat germ cells could proliferate and complete meiosis in a three-dimensional (3D) culture system, while murine male germ cells underwent differentiation and formed spermatozoa in soft agar, and functional sperm was produced in neonatal mouse testis tissue cultured in vitro [11820]. Recently, it was reported that haploid cells were generated from embryonic stem cell-derived germ cells with the presence of retinoic acid (RA), bone morphogenetic protein (BMP), activin A, follicle-stimulating hormone (FSH), and testosterone (T) [30]. Additionally, in vitro differentiation of human SSCs has also been attempted and haploid spermatids were derived from human SSCs [26]. However, in vitro spermatogenesis in domestic animals has been rarely reported. Previous study has shown that spermatids can be generated from bovine type A spermatogonia during long-term cultivation; however, markers related to meiosis were not detected [15]. A recent study reported that functional haploid cells were generated from male germ cells of goat [7]. At present, studies on in vitro spermatogenesis from pig SSCs are progressing slowly.
To date, the spermatogenesis mechanism has been incompletely described. A previous study demonstrated that RA was a key factor in the initiation of meiosis [5]. The addition of FSH and T into culture medium can prevent male germ cells from undergoing apoptosis and can promote SSC differentiation [822]. In addition, activin A was reported to have an important role in spermatogenesis [19], and a previous study reported that BMP4 was required for self-renewal of germ cells [14].
In the present study, testicular cells of Bama mini-pig were co-cultured in medium along with added growth factors and hormones in order to initiate spermatogenesis and explore the differentiation capability of testicular cells into late-stage male germ cells.
Study animals were handled in compliance with the Animal Care and Use Committee of the Germplasm Resource Center of Chinese Experimental Mini-pig and Animal Care and Use Committee of Guangxi University (approval No. GXU2016-015). Bama mini-pigs were obtained from the Animal Experiment Center of Guangxi University. The pigs were bred in an enclosed barn at 20℃ and fed according to their requirements. To obtain testes, pig scrotum was cleaned with water, sterilized with 75% alcohol, and incised with a scalpel. To avoid microorganism contamination, the testes were then sterilized with 75% alcohol for 10 min and washed three times in phosphate buffered saline (PBS, pH 7.2). The testicular cells were evaluated for contamination with mycoplasma by using Hoechst 33342 staining; the results indicated mycoplasma-free cultures.
The 1-month-old (1-mo) and 2-month-old (2-mo) testis tissues were fixed in Bouin's fixative for 12 h, rinsed in water for 2 h, dehydrated, embedded in paraffin, and then sectioned (4 µm thick). The sections were stained with hematoxylin and eosin in sequence, dehydrated, mounted, and finally imaged.
The 1-mo testis was decapsulated and minced, suspended in minimum essential media alpha (α-MEM; Life Technologies, USA) containing collagenase (1 mg/mL), hyaluronidase (1.5 mg/mL) and DNase I (5 µg/mL), cultured for 40 min at 37℃, and filtered sequentially through 70 µm and 40 µm cell strainers. Isolated cells were seeded in six kinds of medium as follows: α-MEM containing 10% Knockout serum replacement (KSR, 10828028; Thermo Fisher Scientific, USA); α-MEM containing 10% fetal bovine serum (FBS; HyClone Laboratories, USA); Dulbecco's modified Eagle medium nutrient mixture F-12 (DMEM/F12, 11330032; Thermo Fisher Scientific) containing 10% KSR; DMEM/F12 containing 10% FBS; Iscove's modified Dulbecco's medium (IMDM, 12440-053; Life Technologies) containing 10% KSR; and IMDM containing 10% FBS. The cells were cultured at 37℃ in a 5% CO2 atmosphere, and the medium was changed every 3 days.
To induce SSC differentiation, 1-mo testicular cells were seeded in two kinds of medium for comparison purposes. Medium 1: α-MEM supplemented with 10% KSR, 3 ng/mL RA, 20 ng/mL BMP4 (R&D Systems, USA), 100 ng/mL activin A (R&D Systems), 200 ng/mL FSH (Sigma, USA) and 10 µM T (Sigma). Medium 2: IMDM supplemented with 10% FBS, growth factors, or hormones, 3 ng/mL RA, 20 ng/mL BMP4 (R&D Systems), 100 ng/mL activin A (R&D Systems), 200 ng/mL FSH (Sigma) and 10 µM T. As controls, the 1-mo and 2-mo testis tissues and the testicular cells were cultured in α-MEM + 10% KSR without growth factors or hormones. Cells were cultured at 37℃ in 5% CO2.
Immunohistochemical staining was performed for SSC characterization in testis tissues, which were fixed in Bouin's fixative for 12 h, rinsed in water for 2 h, dehydrated, embedded in paraffin, and then sectioned. Tissue sections were dewaxed, rehydrated, boiled in 10 mM sodium citrate (pH 6.0) for 30 min, washed in PBS (pH 7.2) three times, cultured with 0.5% Triton X-100 for 5 min, blocked with 5% bovine serum albumin (BSA) in PBS for 30 min. Dual staining was performed as follow: sections were incubated with rhodamine-labeled Dolichos biflorus agglutinin (DBA) (1:100 dilution; Vector Laboratories, USA) and rabbit anti-human protein gene product 9.5 (UCHL1, 1:200 dilution; AbD Serotec, UK) for 3 h, rinsed with PBS, and cultured in donkey anti-rabbit secondary antibody (1:500 dilution; Invitrogen Molecular Probes, USA) for 15 min. Hoechst 33342 staining was performed to visualize the nuclei. Primary antibodies were replaced with 1% BSA in PBS as a negative control.
The testicular cell colonies were characterized with primary antibodies including rabbit anti-Oct4 (1:100 dilution; Abcam, USA), rabbit anti-human UCHL1 (1:200 dilution), rabbit anti-human PLZF (1:50 dilution; Santa Cruz Biotechnology, Germany), rabbit anti-rat THY1 (1:100 dilution; Abbiotec, USA), and rhodamine-labeled DBA (1:100 dilution). Briefly, cell colonies were fixed in 4% paraformaldehyde, rinsed in PBS, penetrated with 0.5% Triton X-100 for 5 min, blocked with 5% BSA in PBS for 30 min, and incubated with primary antibodies for 3 h at room temperature. Primary antibodies were replaced with 1% BSA in PBS as a negative control. Sections were rinsed in PBS and incubated with donkey anti-rabbit secondary antibody (1:500 dilution; Invitrogen Molecular Probes) for 15 min. Hoechst 33342 staining was performed to visualize the nuclei. As a final step, the slides were washed and mounted.
Cells undergoing induction were identified by using antibodies, including rabbit anti-human SCP3 (1:100 dilution; Abcam) and rabbit anti-mouse Stra8 (1:200 dilution; Abcam). Immunohistochemistry staining is performed to identify the colonies.
The level of AP activity in tissue and colonies was determined by using the Alkaline Phosphatase Substrate Kit (SK-5300; Vector Laboratories) following the steps recommended by the manufacturer.
Cultured cells and testicular tissues from 1-mo and 2-mo Bama mini-pig were used for qRT-PCR analysis. Total RNA was extracted by using TRIzol Reagent (Invitrogen Molecular Probes) according to the manufacturer's instructions. The concentration and purity of the total RNA were determined by using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific). Subsequently, 1 µg of total RNA was reverse-transcribed into first strand cDNA by using the PrimeScript RT reagent kit along with gDNA Eraser (RR047A; Takara, China) following the manufacturer's instructions.
The qRT-PCR was performed by using SYBR Premix Ex Taq II (Tli RNaseH Plus, RR820A; Takara) and a LightCycler 96 instrument (Roche Diagnostics, Switzerland). The total 20 µL reaction volume was composed of SYBR Premix Ex Taq II (10 µL), forward primer (1 µL, 2 µM), reverse primer (1 µL, 2 µM), cDNA (2 µL), and double distilled H2O (6 µL). The qPCR reaction was conducted as follows: 95℃ for 30 sec, 40 cycles of 95℃ for 5 sec, and 60℃ for 30 sec (acquisition mode: single). Melting curve analysis was conducted according to instrument-specific procedures. The relative quantitative data were calculated by using the 2−ΔΔCt method, all values were normalized to the house-keeping gene GAPDH. The primers used for qRT-PCR are listed in Table 1.
In the prepared histological sections of the testes, the SSCs localized on the basement membrane of the seminiferous tubules exhibited a high nucleus-to-cytoplasm ratio (panel A in Fig. 1; asterisk) in 1-mo testis; no spermatids or sperm were observed. These observations indicated that spermatogenesis did not initiate in Bama mini-pig tissue at the age of 1 month. However, spermatogenesis was initiated in 2-mo testis, because spermatid (panel B in Fig. 1; arrowhead) and elongated sperm (panel B in Fig. 1; arrow) were observed within the seminiferous tubules.
The cells were seeded in dishes and maintained in different culture systems for 10 days without passage, colony formation varied among the culture systems (Fig. 2). 3D colonies were observed when the testicular cells were cultured in α-MEM + 10% KSR; the cells proliferated robustly and aggregated together 3 days after seeding (panel A in Fig. 3); flat colonies formed after 4 to 5 days of culture (panel B in Fig. 3), and they grew continuously and became compact by 6 days of culture (panel C in Fig. 3); finally, 3D colonies were formed and individual cells could not be distinguished (panel D in Fig. 3). No colony was formed in α-MEM + 10% FBS culture. Loosely organized colonies with unclear borders were observed when the cells were cultured in DMEM/F12 + 10% KSR, and no colony formation was observed in DMEM/F12 + 10% FBS culture. When the testicular cells were cultured in IMDM supplemented with KSR or FBS, they grew as fibroblast-like cells, and there was no colony formation.
Dual staining was performed for SSC identification in testis tissue sections. UCHL1 expression was observed in SSCs located at the basement membrane of seminiferous tubules; however, the expression level, measured as fluorescence intensity, varied (panel A in Fig. 4). DBA-positive staining was detected in SSCs in which UCHL1 expression level was high (panel B in Fig. 4), whereas SSCs with low-level expression of UCHL1 showed DBA-negative staining (panel D in Fig. 4). AP staining showed that the SSCs in testis had AP activity (panel E in Fig. 4).
Immunocytochemical staining showed that the cultured colonies expressed SSC markers including UCHL1, THY1, OCT4, and PLZF, and they were positive for DBA staining; the cells in the center of the colonies showed AP-positive staining (Fig. 5).
Bama mini-pig testicular cells were cultured using different systems. We observed that cell colonies were formed under α-MEM + KSR culture, whereas no colony was formed when cells were cultured in IMDM supplemented with FBS or KSR. As the optimal culture conditions for Bama mini-pig spermatogenesis have not been fully described, we compared the induction effects on cells cultured in α-MEM + KSR and IMDM + FBS supplemented with induction factors.
When the testicular cells were cultured in differentiation medium, no colony formation occurred. At 10 days after induction, cells were collected for analysis. Immunocytochemical staining analysis showed that stimulated by RA gene 8 (Stra8) was detected in the cytoplasm of germ cells when testicular cells were cultured in Medium 1 and Medium 2. Moreover, synaptonemal complex protein 3 (SCP3) was detected in the nuclei of germ cells in both media (Fig. 6).
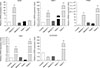
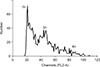
We next compared the mRNA expression levels of spermatogenesis-associated genes in the cells cultured in differentiation media with testes from 1-mo and 2-mo pigs. The qRT-PCR analysis showed that Stra8 expression was significantly upregulated (p < 0.01) in cells cultured in Medium 1 compared to that in the control 1-mo and 2-mo testes, whereas it was downregulated when the cells were cultured in Medium 2. Furthermore, expression levels of DMC1, PRM1, and TNP1 were significantly upregulated (p < 0.01) in cells cultured in Medium 1 and only slightly upregulated when cultured in Medium 2 when compared to the control and 1-mo testis, while they were significantly downregulated compared to the levels in the 2-mo testis. Additionally, the mRNA levels of acrosin in cells cultured in Medium 1 and Medium 2 were both downregulated, though not significantly, compared that of the control. However, the acrosin levels were significantly (p < 0.01) low compared to that in the 2-mo testis (Fig. 7). In addition, DNA content was determined by using flow cytometry. A haploid peak was detected in the analysis with the results indicating that haploid cells were formed (Fig. 8).
Spermatogenesis is a complicated multi-step process occurring within the seminiferous tubules of testes, and that process is regulated by multiple factors associated with germ cells and other testicular cells, such as Sertoli cells and Leydig cells. In vitro proliferation of SSCs is a prerequisite for the study of differentiation, and several attempts have been made to establish a culture system for SSCs. It has been reported that bovine SSC colonies were formed on extracellular matrix-coated plates [2]. Putative SSCs from cat have been isolated and then maintained for 57 days in vitro [23], while goat SSCs were cultured for only 2 weeks [13]. Porcine testicular cells were cultured in vitro and porcine SSC-like colonies could be maintained for 9 passages [17]. Previously, we reported that SSCs of Bama mini-pig could be maintained for up to 3 months on SIM mouse embryo derived thioguanine- and ouabain-resistant (STO) feeder layer [28]. However, the cultured SSCs were influenced by unknown factors from serum and feeder layer cells, and no previous report has provided evidence that long-term-cultured SSCs from pig can maintain spermatogenesis potential. Thus, in this study, primary cells from pig testis were co-cultured with various factors to mimic the environment of spermatogenesis in vivo.
Before the induction study, we investigated the appropriate culture medium for SSCs proliferation. DMEM/F12 and FBS are commonly used for SSCs expansion [41112], and α-MEM supplemented with KSR was used for mouse SSC proliferation [3]. Recently, a study reported that mouse SSCs could be maintained for a long term in a novel culture system containing IMDM and KSR [16]. Previous reports did not elucidate what culture medium was suitable for pig SSC proliferation. Therefore, in this study, six kinds of medium were tested for their effects on testicular cells proliferation. The culture results showed that colonies containing SSCs were formed in some media, while no colonies were formed in others. However, we could not determine which culture conditions were optimal for in vitro differentiation. Thus, induction studies were performed in α-MEM and KSR, and IMDM and FBS.
Stra8, the downstream gene regulated by RA, was significantly upregulated when testicular cells were cultured in α-MEM supplemented with KSR and induction factors. This result indicated that RA could trigger meiosis in this medium [29]. SCP3 and DMC1 are meiosis-specific markers [624]. During spermatogenesis after meiosis, histones are replaced by protamines in the nuclear region of spermatids. Protamine 1 (PRM1) has been detected in all mammals examined, and protamine 2 (PRM2) has been observed in a few domestic animals [21]. It has been suggested that transition nuclear protein 1 (TNP1), an essential factor present in the condensing chromatin of spermatids, participates in nuclear condensation [27]. In the present study, mRNA expression levels of DMC1, PRM1, and TNP1 were all upregulated significantly. These results suggested that SSCs underwent differentiation and developed to a post-meiotic stage with the addition of BMPs, activin A, FSH, and T. These results confirmed those in a previous study [5]. ACROSIN encodes as an enzyme in the head of elongated sperm, and its expression level was not upregulated in this study [10], indicating that transformation of spermatids had not occurred or was incomplete.
Different results were observed when the cells were induced in IMDM supplemented with FBS and the tested induction factors. Under those induction conditions, Stra8 was downregulated. Consistently, the expression levels of genes relative to spermatogenesis were not upregulated significantly. The results indicated that RA could not trigger spermatogenesis under the tested conditions. This result was different from those in a previous study, which suggested that FBS could enhance meiosis when combined with RA, T, and FSH [25]. Therefore, we speculate that unknown factors in IMDM can inhibit the function of RA on SSCs.
In summary, through this study, we have established a differentiation system suitable for pig SSCs in which the SSCs can undergo differentiation and develop to the post-meiotic stage.
Figures and Tables
Fig. 1
Histology of testis tissues. (A) Image of a 1-month-old testis. Spermatogonial stem cells were observed (asterisk), but no spermatids or sperm were observed. (B) Image of a 2-month testis. Round spermatids (arrowhead) and elongated sperm (arrow) were observed. Scale bars = 50 µm (A and B).

Fig. 2
Cultivation of testicular cells in vitro. Three-dimensional colonies were formed when testicular cells were cultured in minimum essential medium alpha (α-MEM) and Knockout serum replacement (KSR), flat colonies were formed when testicular cells were cultured in Dulbecco's modified Eagle medium nutrient mixture F-12 (DMEM/F12) and KSR. IMDM, Iscove's modified Dulbecco's medium; FBS, fetal bovine serum. Scale bars = 100 µm.
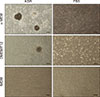
Fig. 3
Colony formation process. After 3 days of culture (A), after 4 to 5 days of culture (B), after 6 days of culture (C), and morphology of colonies observed as finally formed (D). Scale bars = 100 µm (A–D).
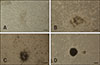
Fig. 4
Characterization of spermatogonial stem cells (SSCs) in 1-month-old testis tissue. SSCs were stained with UCHL1 (A) and double stained with Dolichos biflorus agglutinin (B). (C) Counterstaining was carried out with Hoechst 33342. (D) Panels A–C in Fig. 4 merged. (E) Alkaline phosphatase staining result. Scale bars = 50 µm (A–E).
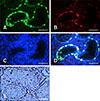
Fig. 5
Identification of the cultured colonies. The colonies expressing UCHL1, PLZF, THY1, and OCT4 showed DBA- and alkaline phosphatase (AP)-positive staining results. Scale bars = 100 µm.
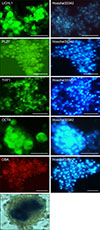
Fig. 6
Immunocytochemical staining of the induced cells. (A) Stra8 expression was observed in the cytoplasm of cells cultured in Medium 1 or Medium 2. (B) Scp3 expression was observed in cell nuclei. Scale bars = 10 µm (A and B).
Fig. 7
Relative expressions of the marker genes involved in spermatogenesis. Relative expression levels of Stra8, DMC1, PRM1, TNP1, and ACROSIN were quantified by quantitative reverse transcription polymerase chain reaction in differentiated cells that were cultured in Medium 1 or Medium 2 compared to those in 1-month testis (Testis 1), 2-month testis (Testis 2), and the control cultures. The average values were from three experiments. The results are shown as mean ± SEM. The data were analyzed by one-way analysis of variance by using PASW Statistics software (ver.18.0; IBM). Double asterisks indicate p < 0.01.
Acknowledgments
This work was supported by the Natural Science Foundation of China (No. 31260277), the Guangxi High Education Innovation Research Group and Eminent Scholar Project, 2017 Excellent Teaching Program of Guangxi High Education - Program of Advantage and Characteristic Specialty (High Quality of Undergraduate Program).
References
1. Abu Elhija M, Lunenfeld E, Schlatt S, Huleihel M. Differentiation of murine male germ cells to spermatozoa in a soft agar culture system. Asian J Androl. 2012; 14:285–293.

2. Akbarinejad V, Tajik P, Movahedin M, Youssefi R, Shafiei S, Mazaheri Z. Effect of extracellular matrix on bovine spermatogonial stem cells and gene expression of niche factors regulating their development in vitro. Anim Reprod Sci. 2015; 157:95–102.

3. Aoshima K, Baba A, Makino Y, Okada Y. Establishment of alternative culture method for spermatogonial stem cells using knockout serum replacement. PLoS One. 2013; 8:e77715.

4. Baazm M, Abolhassani F, Abbasi M, Habibi Roudkenar M, Amidi F, Beyer C. An improved protocol for isolation and culturing of mouse spermatogonial stem cells. Cell Reprogram. 2013; 15:329–336.

5. Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science. 2006; 312:596–600.

6. Bukovsky A, Caudle MR, Gupta SK, Svetlikova M, Selleck-White R, Ayala AM, Dominguez R. Mammalian neo-oogenesis and expression of meiosis-specific protein SCP3 in adult human and monkey ovaries. Cell Cycle. 2008; 7:683–686.

7. Deng S, Wang X, Wang Z, Chen S, Wang Y, Hao X, Sun T, Zhang Y, Lian Z, Liu Y. In vitro production of functional haploid sperm cells from male germ cells of Saanen dairy goat. Theriogenology. 2017; 90:120–128.

8. Erkkilä K, Henriksén K, Hirvonen V, Rannikko S, Salo J, Parvinen M, Dunkel L. Testosterone regulates apoptosis in adult human seminiferous tubules in vitro. J Clin Endocrinol Metab. 1997; 82:2314–2321.

9. Feng LX, Chen Y, Dettin L, Pera RA, Herr JC, Goldberg E, Dym M. Generation and in vitro differentiation of a spermatogonial cell line. Science. 2002; 297:392–395.

10. Ferrer M, Rodriguez H, Zara L, Yu Y, Xu W, Oko R. MMP2 and acrosin are major proteinases associated with the inner acrosomal membrane and may cooperate in sperm penetration of the zona pellucida during fertilization. Cell Tissue Res. 2012; 349:881–895.

11. Fujihara M, Kim SM, Minami N, Yamada M, Imai H. Characterization and in vitro culture of male germ cells from developing bovine testis. J Reprod Dev. 2011; 57:355–364.

12. Goel S, Sugimoto M, Minami N, Yamada M, Kume S, Imai H. Identification, isolation, and in vitro culture of porcine gonocytes. Biol Reprod. 2007; 77:127–137.
13. Heidari B, Rahmati-Ahmadabadi M, Akhondi MM, Zarnani AH, Jeddi-Tehrani M, Shirazi A, Naderi MM, Behzadi B. Isolation, identification, and culture of goat spermatogonial stem cells using c-kit and PGP9.5 markers. J Assist Reprod Genet. 2012; 29:1029–1038.

14. Hu J, Chen YX, Wang D, Qi X, Li TG, Hao J, Mishina Y, Garbers DL, Zhao GQ. Developmental expression and function of Bmp4 in spermatogenesis and in maintaining epididymal integrity. Dev Biol. 2004; 276:158–171.

15. Izadyar F, Den Ouden K, Creemers LB, Posthuma G, Parvinen M, De Rooij DG. Proliferation and differentiation of bovine type A spermatogonia during long-term culture. Biol Reprod. 2003; 68:272–281.

16. Kanatsu-Shinohara M, Ogonuki N, Matoba S, Morimoto H, Ogura A, Shinohara T. Improved serum- and feeder-free culture of mouse germline stem cells. Biol Reprod. 2014; 91:88.
17. Kuijk EW, Colenbrander B, Roelen BA. The effects of growth factors on in vitro-cultured porcine testicular cells. Reproduction. 2009; 138:721–731.

18. Lee JH, Kim HJ, Kim H, Lee SJ, Gye MC. In vitro spermatogenesis by three-dimensional culture of rat testicular cells in collagen gel matrix. Biomaterials. 2006; 27:2845–2853.

19. Mithraprabhu S, Mendis S, Meachem SJ, Tubino L, Matzuk MM, Brown CW, Loveland KL. Activin bioactivity affects germ cell differentiation in the postnatal mouse testis in vivo. Biol Reprod. 2010; 82:980–990.

20. Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, Kubota Y, Ogawa T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011; 471:504–507.

21. Takeda N, Yoshinaga K, Furushima K, Takamune K, Li Z, Abe S, Aizawa S, Yamamura K. Viable offspring obtained from Prm1-deficient sperm in mice. Sci Rep. 2016; 6:27409.

22. Tesarik J, Greco E, Mendoza C. Assisted reproduction with in-vitro-cultured testicular spermatozoa in cases of severe germ cell apoptosis: a pilot study. Hum Reprod. 2001; 16:2640–2645.

23. Tiptanavattana N, Techakumphu M, Tharasanit T. Simplified isolation and enrichment of spermatogonial stem-like cells from pubertal domestic cats (Felis catus). J Vet Med Sci. 2015; 77:1347–1353.

24. Wang H, Sun M, Qin Y, Xia T, Ma J, Chen ZJ. Mutations in DMC1 are not responsible for premature ovarian failure in Chinese women. Reprod Biomed Online. 2013; 26:175–178.

25. West FD, Mumaw JL, Gallegos-Cardenas A, Young A, Stice SL. Human haploid cells differentiated from meiotic competent clonal germ cell lines that originated from embryonic stem cells. Stem Cells Dev. 2011; 20:1079–1088.

26. Yang S, Ping P, Ma M, Li P, Tian R, Yang H, Liu Y, Gong Y, Zhang Z, Li Z, He Z. Generation of haploid spermatids with fertilization and development capacity from human spermatogonial stem cells of cryptorchid patients. Stem Cell Reports. 2014; 3:663–675.

27. Yu YE, Zhang Y, Unni E, Shirley CR, Deng JM, Russell LD, Weil MM, Behringer RR, Meistrich ML. Abnormal spermatogenesis and reduced fertility in transition nuclear protein 1-deficient mice. Proc Nat Acad Sci USA. 2000; 97:4683–4688.

28. Zhao HM, Yang H, Luo FH, Li MX, Zhang S, Yang XG, Lu YQ, Lu SS, Wu YJ, Lu KH. Isolation, proliferation, and induction of Bama mini-pig spermatogonial stem cells in vitro. Genet Mol Res. 2016; 15:15038602.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download