Abstract
This study describes ultrasonographic observations of five hepatobiliary diseases in buffalo (Bubalus bubalis). Fifty buffalo, including 20 clinically normal and 30 hepatobiliary diseased buffalo were enrolled in the study. Complete clinical, radiographic and ultrasonographic examinations and laboratory analyses were conducted. Focal parenchymal lesions including liver abscess (n = 12) and hepatic cyst (n = 6), diffuse parenchymal lesion (hepatobiliary cirrhosis, n = 5) and obstruction of hepatobiliary passages including cholestasis (n = 4), and hepatocholelithiasis (n = 3) were successfully imaged by ultrasonography. Hepatic abscess imaged as a hypoechoic to echogenic circumscribed mass of various diameters with a distinct echogenic capsule. Hepatic cyst imaged as a pear-shaped sac with a bright echogenic margin, anechoic content, and distal acoustic enhancement. In hepatobiliary fibrosis, the liver showed linear bands of increasing echogenicity with less distinct imaging of the portal vasculature. Cholestasis was imaged as dilatation of the gallbladder (GB) with wall thickening and homogeneous or heterogeneous contents. Hepatocholelithiasis imaged as an echoic structure within the hepatic parenchyma, or within and around the GB and bile duct, with more echogenicity of the hepatic parenchyma than normal. Ultrasonography can be an efficient rapid, noninvasive tool for screening of common hepatobiliary diseases in buffalo under field conditions.
Hepatic diseases are of great economic importance in bovine medicine [1127]; however, they receive little attention because they may be unnoticed due to their nonspecific clinical signs [11]. Abattoir-based survey studies are commonly conducted for detection of liver afflictions [8] due to difficulties reaching an accurate diagnosis in clinic-based situations. Additionally, laboratory-based diagnosis of hepatobiliary diseases, such as that from hepatospecific enzyme tests, is insufficient and time consuming [28].
The negative effects and economic losses of hepatobiliary diseases on animal's productivity have been documented; moreover, affected livers are condemned at slaughter, and adhesions to the surrounding organs or diaphragm may necessitate carcass trimming [27].
Recent advances in diagnostic tools and the introduction of ultrasonic imaging have facilitated screening of different diseases of internal organs, including hepatic diseases in buffalo under field conditions [35]. A complete ultrasonographic examination of the liver can provide detailed information about the nature of an affliction, the parenchymal pattern of the liver, the size and position of the gallbladder (GB), and the intra- and extrahepatic bile ducts in cattle [11] and buffalo [18].
Both focal and diffuse liver afflictions including abscesses, neoplasia, cyst, and cirrhosis, as well as hepatobiliary diseases such as cholangitis and cholelithiasis, have been recorded separately in various animal species [1]. These afflictions can develop as primary diseases or as sequelae to other diseases such as traumatic reticuloperitonitis (TRP) [567]. They are commonly reported in feedlot and dairy cattle being fed rations that predispose to rumenitis [26].
In recent years, ultrasonography has become an increasingly important tool for diagnosis of various abdominal disorders in bovine practice [9]. Several studies concerned with ultrasonography of digestive system disorders have been carried out in buffalo, particularly studies into TRP [241922] and intestinal disorders [2021], and cattle [2429]. However, there are few studies describing ultrasonographic results associated with hepatobiliary diseases in buffalo [1]. Therefore, this research was undertaken to document ultrasonographic observations of naturally developed hepatobiliary diseases in buffalo and relate those results to results from clinical and biochemical assessments.
The diseased group included 30 Egyptian buffalo (Bubalus bubalis; aged 7 months to 8 years) of both sexes were admitted to the Veterinary Teaching Hospital (VTH) at Assiut University (Egypt) with histories of weight loss or poor growth rate, as well as reduction of milk yield in female animals. Some cases had a history of long-standing diarrhea. A control group (n = 20) was selected from a group of healthy, non-pregnant female buffalo in a herd at the VTH.
All animal procedures performed in this study were conducted in accordance with Institutional Animal Care and Use Committee guidelines of Assiut University which basically conform to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health in the USA (NIH publication No. 86-23, revised 1996).
All animals underwent a thorough clinical examination as described by Cockcroft [16].
Left lateral recumbent plain radiographs of the cranial, abdominal, and caudal thoracic regions were obtained from both control and hepatobiliary diseased buffalo. The examination was carried out according to the method reported by Braun et al. [12] by using a ceiling-suspended X-ray apparatus (45–65 Kv and 45–55 mA/s). The following features were recorded: foreign body nature and location (reticular, diaphragmatic, or pericardial position), diaphragm status, and heart visualization (good versus bad line of demarcation).
Hepatic ultrasonography was performed according to the methods reported by Braun [11] and Khalphallah et al. [18] by using 3.5 MHz sector transducer connected with the ultrasound device (FF Sonic UF-400; Fukuda, Japan). In preparation for ultrasonography, the right thorax and abdomen areas were clipped and shaved, and a coupling gel was applied. Ultrasonographic examination of the liver was performed on the right side of the abdomen in the region of the 7th to 12th intercostal spaces (ICSs). The circumferences of the liver and GB were determined. The nature of hepatic lesions, focal or diffuse, and observed disorders in the biliary system were recorded. The left thorax area and the area behind the xiphoid process were also prepared and screened for TRP according to the method reported by Braun and Götz [13].
Whole blood and serum samples were collected, and all precautions for sample collection and preparation to ensure accurate evaluation of hematological and biochemical indices, as described by Coles [17], were taken into consideration.
A fully automated blood cell counter machine (CA620 Vet Hematology Analyzed; Medonic, Sweden) was used to determine various hematological parameters. The differential leukocytic count was determined by using the four-field meander method.
Serum concentrations of aspartate aminotransferase (AST), γ-glutamyltransferase (GGT), alkaline phosphatase (ALK), total protein, albumin, cholesterol, and triglyceride (TG) were assessed by using the Optizen32220 UV/Visible spectrophotometer (Mecasys, Korea). Serum globulin was determined by subtracting albumin level from total protein level.
All kits and reagents were obtained from Spectrum Reagents (Egyptian Company for Biotechnology, Egypt).
Data were analyzed by using the SPSS statistical software packaged program for Windows (ver. 10.0.1; SPSS, USA). Data are presented as mean ± SD values. Analysis of the variance of the obtained data was performed by using Student's t-test and significance level of results was set at p ≤ 0.05.
Most of the hepatobiliary diseased buffalo (21/30) had a poor body condition score (BSC < 2) (panel A in Fig. 1) and a history of inappetence. Some cases (9/30) experienced long-standing diarrhea. Body temperature and respiratory rate were normal in most diseased buffalo (28/30); however, ruminal movement was greatly reduced. The visible mucous membranes, including the conjunctiva, were pale and rarely (2/30) icteric. The episcleral blood capillaries were empty in most diseased buffalo. Six buffalo with hepatobiliary afflictions associated with TRP and/or traumatic pericarditis (TP) showed anorexia, tucked up appearance, tensed abdomen, and recurrent tympany.
In both the control buffalo and most of the hepatobiliary diseased buffaloes (24/30), radiography showed an absence of any reticular metal objects. The diaphragm was imaged clearly between two radio-opaque structures; reticulum and heart. The heart appeared as radio-opaque with clear margins, normal size, and a characteristic shape (cone shape with base of the heart directed dorsally and apex directed ventrally).
Radiography of buffalo with hepatobiliary diseases associated with TRP and/or TP (n = 6) showed different radio-opaque metallic foreign bodies (needles, hair-pins, and wires) within the reticulum in buffalo with normal heart (n = 4) or affected heart (n = 2; panel B in Fig. 1).
Five types of hepatobiliary afflictions were successfully imaged in the hepatobiliary diseased buffalo (Table 1). Based on ultrasonographic results, these afflictions included focal parenchymal lesions including liver abscess and cyst in 18 of the 30 diseased buffalo. Hepatic abscess, recorded in 12 (40.0%) of the diseased buffalo, imaged as a well-demarcated focal mass with an echogenic capsule and hypoechoic or anechoic contents producing distal acoustic shadowing (Fig. 2). Sometimes (2/12), hepatic abscess was associated with visualization of an echogenic clear tract within the hepatic tissue extending from the abscess and with accumulation of thick echogenic deposits around the GB, abscess, cystic duct, and common hepatic duct (i.e., pericholecystic hyperechogenic material).
Buffalo with hepatic abscess associated with TRP and/or TP (n = 6) showed a thick wall reticulum with uneven contour, reduced reticular contractions with displacement of reticulum away from diaphragm, peritoneal effusions, reticular abscess, and spleen involvement. Reticular abscess was imaged as a well-distinguished echogenic capsule with hypoechoic content. The involved spleen was completely covered with a fibrinous echogenic deposit and was surrounded with hypoechoic fluid associated with echogenic fibrinous deposits leading to adhesions between spleen and the abdominal wall, as well as between spleen and reticulum and/or the craniodorsal blind sac of the rumen. In cases with TP (n = 2), cardiomegaly with loss of the characteristic cardiac shape, thickened cardiac wall, and accumulation of pericardial hypoechoic fluids interspersed with echogenic deposits was also imaged.
Liver cyst was recorded in 6 buffalo representing 20% of the total diseased buffalo. Liver cyst appeared as a pear-shaped anechoic sac with a well-defined thin bright echogenic wall and a peripheral refractive zone situated ventromedially to the liver causing distal acoustic enhancement (Fig. 3).
Diffuse parenchymal lesion (hepatobiliary fibrosis or liver cirrhosis) was diagnosed in 5 buffalo representing 16.7% of the 30 diseased buffalo. The liver showed echogenic linear bands with less distinct imaging of the portal vasculature. Multiple echogenic nodules and anechoic foci on the liver were imaged, giving the liver a heterogeneous nature. Fibrinous echogenic deposits were visualized around the caudal vena cava, resulting in reduction of its lumen (Fig. 4). Liver size was reduced, and liver could not be imaged from the right 12th ICS. Ultrasonography also was used to diagnose complete hepatobiliary cirrhosis with a dilated loop of the small intestine. The dilated loop of the cranial part of the duodenum (6.2–6.5 cm) was intertangled medially to the GB and liver (Fig. 5).
Obstruction of hepatobiliary passages was diagnosed in 7 buffalo representing 23.3% of the total diseased buffalo. The obstructions included cholestasis (n = 4) and hepatocholelithiasis (n = 3).
Cholestasis was recorded in 4 buffalo representing 13.3% of the total diseased buffalo. Dilatation of GB with thickening in its wall, dilatation of the bile duct and cystic duct, alterations in GB content (homogeneous or heterogeneous), and hepatomegaly were the most common ultrasonographic observations in buffalo with cholestasis (Fig. 6).
Hepatocholelithiasis was reported in 3 buffalo representing 10% of the total diseased buffalo. It appeared as an echoic structure inside the hepatic parenchyma or inside and around the GB and bile duct. In addition, there was greater echogenicity of the hepatic parenchyma than that of normal parenchyma (Fig. 7). Distal acoustic shadowing was also observed.
Results of blood and serum biochemical analyses of buffalo with confirmed hepatobiliary diseases are summarized in Table 2. Compared to the control buffalo, the diseased animals had a significantly decreased (p < 0.05) RBC count, lower packed cell volume, and lower hemoglobin concentration with neutrophilic leucocytosis. Blood results in buffalo with hepatobiliary diseases associated with TRP and/or TP showed lymphocytic leucocytosis. Serum biochemical analysis of buffalo with either hepatobiliary disease only or hepatobiliary disease associated with TRP and/or TP showed a significant (p < 0.05) decrease in serum total protein (hypoproteinemia) and albumin (hypoalbuminemia) from levels in control buffalo. Significant (p < 0.05) elevations of blood serum levels of GGT, ALK, and AST were present in hepatobiliary diseased buffalo; however, serum levels of TG and cholesterol were not significantly different between the groups.
The clinical signs associated with hepatobiliary diseases in cattle and buffalo are too vague to allow specific diagnosis, and hence, there are no reports of successful treatment. Cattle may carry hundreds of small abscesses or several large abscesses with no clinical signs, and the abscesses are detected only at the time of slaughter [1141523]. Therefore, the present study was designed to describe the most common ultrasonographic observations in hepatobiliary diseased buffalo. The present results show that liver ultrasonography is an efficient tool for diagnosis of five hepatobiliary diseases: hepatic abscess and cyst, liver cirrhosis, cholestasis, and hepatocholelithiasis.
In this study, buffalo with hepatobiliary diseases showed no specific clinical signs, such as poor BSC, long-standing diarrhea, pale and rarely icteric mucous membranes, or empty episcleral blood capillaries. These results are in agreement with results of previous studies in cattle [123].
Blood composition and chemistry of the hepatobiliary diseased buffalo showed the presence of neutrophilic leucocytosis, hypoproteinemia, and significant elevation of the serum enzymes GGT, ALK, and AST. Although these indices may suggest liver dysfunction, they are nonspecific for diagnosis of hepatobiliary diseases. Similar observations have been reported previously [1].
Ultrasonographic examination of the liver is recommended when buffalo have poor production and/or exhibit weight loss in the absence of specific clinical signs because such examination is rapid, noninvasive, and accurate for the diagnosis of several abdominal disorders [25]. Moreover, radiographic examination of the caudal thoracic and cranial abdominal regions is also recommended whenever TRP and/or TP are suspected.
Liver abscess images showed well-demarcated focal masses within the normal hepatic parenchymal tissue and revealed echogenic capsule with contents of various echogenicity according to the consistency of the pus. Similar observations have been reported in cattle [15]. In this study, ultrasonography of liver abscess with TRP and spleen involvement revealed complete reduction of reticular contraction with displacement of reticulum away from the diaphragm along with imaging of peritoneal effusions and reticular abscess. A similar description of TRP with spleen involvement was reported in cows [12].
Ultrasonographic observation showed that liver cysts appear as black pear-shaped sacs with a bright echogenic margin and a well-defined thin wall within normal hepatic parenchymal tissue producing a peripheral refractive zone. Khalphallah et al. [23] reported similar observations in cattle.
Hepatobiliary cirrhosis was easily diagnosed by ultrasound in buffalo. In such cases, the liver did not extend caudal to the last rib, indicating a reduction of liver size. The cirrhotic liver showed linear echogenic bands and indistinct imaging of the portal vasculature. These results are similar to those reported for cattle [23]. Failure of visualization of portal vasculature is due to the heterogeneous nature of hepatic parenchyma with accumulation of echogenic fibrinous deposits and hepatic parenchymal bands. A complicated case of liver cirrhosis with proximal ileus was diagnosed in the present study. The characteristic ultrasonographic observation, in that case, was the presence of dilated small intestine loops related to liver and GB. Similar observations have been previously recorded in cattle [14] and buffalo [20].
Hepatobiliary obstruction in buffalo, including cholestasis and hepatocholelithiasis, was also diagnosed by ultrasonography in this study. Buffalo with cholestasis showed GB dilatation with thickened GB wall and altered contents (homogeneous or heterogeneous). Cholestasis may be associated with hepatitis or hepatic degeneration. In the absence of liver changes, the main cause of cholestasis is mechanical biliary obstruction by calculi, biliary cirrhosis, cholangitis, and cholecystitis, as has been reported previously in cows [1423].
Hepatocholelithiasis presented as echoic structures within the hepatic parenchyma, or within and around GB and bile duct, with increased echogenicity of the hepatic parenchyma than that observed in normal parenchyma. Dilatations of the bile duct, cystic duct, and GB, which had a thickened wall and altered contents, were also observed. These results are essentially similar to those reported in previous studies of cattle with hepatocholelithiasis [1023].
This study has several limitations, including its small sample size and difficulties in performing follow up of diseased buffalo after slaughter; consequently, there was a lack of both gross and histopathological examination results to confirm the diagnosed hepatobiliary diseases. Regardless, we conclude that ultrasonography can be used effectively as a rapid noninvasive tool for screening of common hepatobiliary diseases in buffalo under field conditions.
Figures and Tables
Fig. 1
(A) A buffalo heifer with traumatic reticuloperitonitis and hepatic abscess and stunted growth. (B) Lateral radiograph of the cranial abdomen and caudal thorax of a 5-year-old non-pregnant buffalo with traumatic pericarditis showing a nail passing from the reticulum and penetrating the heart. a, heart; b, reticulum; c, diaphragm; d, nail; e, loss of heart details.

Fig. 2
Ultrasonograms of 1-year-old buffalo heifer (left) and 1-year-old buffalo bull (right) showing liver (b) with hepatic abscess (c) when imaged from the right 9th, 10th, and 11th intercostal space (ICS). Right abdominal wall (a). Liver abscess was imaged as well a distinct echogenic capsule and hypoechoic lumen. An echogenic clear tract (d) was imaged within the hepatic tissue with accumulation of echogenic deposits around the gallbladder and the abscess. The abscess formed a thick layer of echogenic deposits around the cystic duct as pericholecystic hyperechogenic material (e). R, right; Cr, cranial; Cd, caudal.
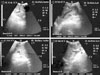
Fig. 3
Ultrasonogram in a 1-year-old non-pregnant buffalo heifer showing liver (b) with hepatic cyst (c) in an image from the right 11th intercostal space (ICS). Right abdominal wall (a). The cyst appears as a pear-shaped structure (black cyst) with bright echogenic margin and a well-defined thin wall located caudoventral to the liver and causing distal acoustic enhancement (d). R, right; Cr, cranial; Cd, caudal.
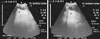
Fig. 4
Ultrasonogram in 2.5, 3, and 4-year-old buffalo bulls obtained at the right 11th and 12th intercostal space (ICS). Right abdominal wall (a). Image shows liver (b) fibrosis/cirrhosis and visualization of linear bands of increasing echogenicity (c) with less distinct visualization of the hepatic and portal vasculatures. Image shows multiple hyperechogenic foci (e) within the hepatic parenchyma with anechoic nodules (d) indicating the heterogeneous nature of the hepatic parenchyma. Reduced lumens of both of caudal vena cava (f) and hepatic vein (g) with accumulation of fibrinous echogenic deposits around the vein are present. Gallbladder (h) and cranial duodenum (i) are also visible. R, right; Cr, cranial; Cd, caudal.
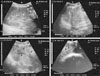
Fig. 5
Ultrasonogram in a 1-year-old buffalo heifer with liver cirrhosis and dilated loops of the cranial duodenum. Images from the right 10th and 11th intercostal space (ICS). Right abdominal wall (a). Multiple hyperechogenic foci (b) located within the hepatic parenchyma with anechoic nodules (c) with a heterogeneous hepatic parenchyma and less distinct visualization of the hepatic and portal vasculatures. Reduced lumens of both of caudal vena cava (d) and hepatic vein with presence of accumulated fibrinous echogenic deposits around the vein. The dilated loop of the cranial part of the duodenum (6.2–6.5 cm; e) was intertangled medially to the gallbladder (f) and liver. R, right; Cr, cranial; Cd, caudal.
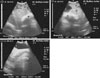
Fig. 6
Ultrasonogram in a 4-year-old non-pregnant buffalo imaged from the right 10th (left) and 12th (right) intercostal spaces (ICSs). Right abdominal wall (a). Image shows healthy liver tissue with normal parenchymal pattern (b) and a dilated gallbladder (cholestasis; c). The dilated gallbladder had a heterogeneous nature with echoic and hypoechoic contents (d), thickening in its wall (e) and distal acoustic enhancement (f). R, right; Cr, cranial; Cd, caudal.
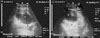
Fig. 7
Ultrasonogram in a 7-month-old fattening buffalo calf imaged from the right 10th intercostal space (ICS). Right abdominal wall (a). Image shows liver (b) with hepatocholelithiasis, which revealed hyperechogenic masses or deposits scattered (c) within the hepatic tissue along with increased hepatic parenchymal echogenicity when compared with normal parenchymal echogenicity of the liver. R, right; Cr, cranial; Cd, caudal.
Acknowledgments
Sincere thanks to the Director of Veterinary Teaching Hospital and the Head of the Department of Animal Surgery at Assiut University for their kind support while conducting this study.
References
1. Abdelaal AM, Gouda SM, Tharwat M. Clinico-biochemical, ultrasonographic and pathological findings of hepatic abscess in feedlot cattle and buffaloes. Vet World. 2014; 7:306–310.

2. Abdelaal AM, Mostafa MB, Abu-Seida AM, Al-Abbadi OS, Abbas SF. Ultrasonographic findings in hardware diseased buffaloes (Babulus babilus). Res J Pharmaceut Biol Chem Sci. 2016; 7:1644–1649.
3. Abu-Seida AM. Current status and prospect of ultrasonographic application in buffaloes. Asian J Anim Vet Adv. 2016; 11:144–157.

4. Abu-Seida AM, Al-Abbadi OS. Recent advances in the management of foreign body syndrome in cattle and buffaloes: a review. Pak Vet J. 2016; 36:385–393.
5. Abu-Seida AM, Al-Abbadi OS. Studies on sharp foreign body syndrome in Iraqi buffaloes and its impact on milk production. Asian J Anim Vet Adv. 2015; 9:128–133.

6. Al-Abbadi OS, Abu-Seida AM, Al-Hussainy SM. Studies on rumen magnet usage to prevent hardware disease in buffaloes. Vet World. 2014; 7:408–411.

7. Andrews AH, Blowey RW, Boyd H, Eddy RG. Bovine Medicine, Diseases and Husbandry of Cattle. 2nd ed. Ames: Blackwell Science;2004. p. 835–839.
8. Berhe G. Abattoir survey on cattle hydatidosis in Tigray Region of Ethiopia. Trop Anim Health Prod. 2009; 41:1347–1352.

9. Blond L, Buczinski S. Basis of ultrasound imaging and the main artifacts in bovine medicine. Vet Clin North Am Food Anim Pract. 2009; 25:553–565.

10. Brandon B, Stanley C. What is your diagnosis? Cholestasis, hepatic cholelithiasis. J Am Vet Med Assoc. 2003; 222:289–290.
11. Braun U. Ultrasonography of the liver in cattle. Vet Clin North Am Food Anim Pract. 2009; 25:591–609.

12. Braun U, Flückiger M, Nägeli F. Radiography as an aid in the diagnosis of traumatic reticuloperitonitis in cattle. Vet Rec. 1993; 132:103–109.

13. Braun U, Götz M. Ultrasonography of the reticulum in cows. Am J Vet Res. 1994; 55:325–332.
14. Braun U, Marmier O, Pusterla N. Ultrasonographic examination of the small intestine of cows with ileus of the duodenum, jejunum or ileum. Vet Rec. 1995; 137:209–215.

15. Braun U, Pusterla N, Wild K. Ultrasonographic findings in 11 cows with a hepatic abscess. Vet Rec. 1995; 137:284–290.

16. Cockcroft PD. Diagnosis and clinical reasoning in cattle practice. In : Cockcroft PD, editor. Bovine Medicine. 3rd ed. Hoboken: John Wiley & Sons;2015. p. 124–132.
17. Coles EH. Veterinary Clinical Pathology. 4th ed. Philadelphia: W.B. Saunders;1986. p. 132–139.
18. Khalphallah A, Abdelhakiem M, Elmeligy E. The ultrasonographic findings of the liver, gall bladder and their related vasculatures in healthy Egyptian buffaloes (Bubalus bubalis). Assiut Vet Med J. 2016; 62:156–162.
19. Khalphallah A, Abu-Seida AM, Abdelhakiem M, Elmeligy E, Mahmoud UT. Laboratory, radiographic and ultrasonographic findings of acute traumatic reticuloperitonitis in buffaloes (Bubalus bubalis). Asian J Anim Vet Adv. 2016; 11:675–683.
20. Khalphallah A, Aref NE, Elmeligy E, El-Hawari SF. Clinical and ultrasonographic observations of functional and mechanical intestinal obstruction in buffaloes (Bubalus bubalis). Vet World. 2016; 9:475–480.

21. Khalphallah A, Elmeligy E, El-Hawari SF, Mahmoud UT. Clinical, laboratory and ultrasonographic findings in Egyptian buffalo (Bubalus bubalis) with caecal and colonic dilatation. Int J Vet Sci Med. 2016; 4:5–10.

22. Khalphallah A, Elmeligy E, Elsayed HK, El-Hawari SF, Elrashidy MH. Diagnostic significance of ultrasonography in complicated traumatic reticuloperitonitis in Egyptian buffaloes (Bubalus bubalis). Asian J Anim Vet Adv. 2016; 11:319–330.
23. Khalphallah A, El-Sebaie A, Raghib M. Cows with heptaobiliary diseases: clinical, biochemical and ultrasonographic findings. Scholar's Adv Anim Vet Res. 2016; 3:56–67.
24. Khalphallah AA, El-Sebaie AH, Raghib MF. Approach for diagnosis of complicated traumatic reticuloperitonitis in cattle using ultrasonography. J Adv Vet Res. 2015; 5:157–164.
25. Lahmar S, Chéhida FB, Pétavy AF, Hammou A, Lahmar J, Ghannay A, Gharbi HA, Sarciron ME. Ultrasonographic screening for cystic echinococcosis in sheep in Tunisia. Vet Parasitol. 2007; 143:42–49.

26. Nagaraja TG, Lechtenberg KF. Liver abscesses in feedlot cattle. Vet Clin North Am Food Anim Pract. 2007; 23:351–369.

27. Radostis OM, Gaym CC, Hinchcliff KW, Constable PD. Veterinary Medicine. A Textbook of The Diseases of Cattle, Horses, Sheep, Pigs and Goats. 10th ed. Philadelphia: Saunders Elsevier;2007. p. 189–138.
28. Tennant BC. Hepatic function. In : Kaneko JJ, Harvey JW, Bruss ML, editors. Clinical Biochemistry of Domestic Animals. 6th ed. New York: Academic Press;2008. p. 379–341.
29. Tesfaye D, Chanie M. Study on rumen and reticulum foreign bodies in cattle slaughtered at Jimma Municipal Abattoir, South West Ethiopia. Am-Euras J Sci Res. 2012; 7:160–167.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download