Introduction
The development, maintenance, and repair of vertebrate nervous systems depend on the activity of a variety of neurotrophins and other molecules [16]. It has been reported that the sciatic nerve has neurotrophic activity, and nerve regeneration, differentiation, and axon outgrowth can be modulated by different sciatic nerve preparations, such as nerve conditioned media [2], nerve explants [8], and nerve secreted fluid collected in regeneration chambers [1]. On the basis of the above reports, in a previous study, we made rat sciatic nerve leachate and observed that it was able to induce the differentiation of rat adipose-derived stem cells into Schwann-like cells [12]. However, the adult rat sciatic nerve is very short and thin. At least 14 adult rats were sacrificed in order to make 100 mL of sciatic nerve leachate in our previous study [12]. In addition, Villegas et al. [24] reported that four rats were needed to make 6 mL of sciatic nerve conditioned media. Although sciatic nerve preparations are effective for inducing neuronal differentiation of stem/progenitor cells, several animals have to be killed to obtain a sufficient quantity of sciatic nerve. Some studies have demonstrated that the role of sciatic nerve preparations in neuronal differentiation depends on neurotrophic factors that Schwann cells secrete such as neuregulin [24] and glypican-1 [16]. Actually, many neurotrophins are highly conserved among different species. Therefore, it may be possible to induce neuronal differentiation of stem/progenitor cells with sciatic nerve leachate from several different animals. To test that speculation, in this study we made a sciatic nerve leachate from sciatic nerves obtained from cattle and examined its effect on neuronal differentiation of rat PC12 cells (a useful cell model for studying neuronal differentiation).
The mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 (MEK/ERK1/2) signaling pathway is often linked with cells survival, proliferation, and differentiation. Previous studies have shown a mechanistic role for MEK/ERK1/2 in the regulation of neurite elongation: the duration of ERK activation determines the switch from proliferation to differentiation [11]. However, whether MEK/ERK1/2 signaling pathway is associated with sciatic nerve preparation-mediated regulation of neuronal differentiation of PC12 cells remains undescribed. Therefore, in this study, our aim was to assess the effect of a cattle sciatic nerve leachate on neuronal differentiation of rat PC12 cells and to detect the underlying mechanisms.
Materials and Methods
Chemicals
Dulbecco's modified Eagle medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco Invitrogen (USA). Horse serum and goat serum were purchased from Hyclone (USA). PD98059 was purchased from Cell Signaling Technology (USA). The β3-tubulin and MAP2 antibodies were acquired from Abcam (England). Antibodies against phosphorylated ERK1/2 (p-ERK1/2) and ERK1/2 antibody were acquired from Santa Cruz Biotechnology (USA).
Preparation of sciatic nerve leachate
Two sciatic nerves (each length 20 cm) were quickly separated with sterile surgical instruments from one cow at a slaughterhouse at Luoyang, China and placed in phosphate buffered saline (PBS). The sciatic nerves were then cut into pieces approximately 2 cm long on a super-clean bench and soaked in 500 mL of culture medium (DMEM) supplemented with 10% FBS and 5% horse serum. After four days, the culture medium was filtered through 100 µm metal mesh to remove the cattle sciatic nerves fragments, and then stored at 4℃.
Cell culture and neuronal induction
Rat pheochromocytoma PC12 cells were obtained from the Cell Bank of the Chinese Academy of Sciences (China) and maintained in high-glucose DMEM supplemented with 10% FBS, 5% horse serum, 100 units/mL of penicillin, and 100 mg/mL of streptomycin at 37℃ in a humidified and 5% CO2 incubator. Culture medium was replaced at a 2/3 ratio every 2 days. For our tests, PC12 cells were seeded in 6-well culture plates at a density of 4 × 104 cells/well (with or without coverslips). Twenty-four hours later, cells were treated with or without cattle sciatic nerve leachate for 9 days; cells exposed to normal medium only were used as controls. For the signaling pathway studies, 10 µM PD98059 (a selective inhibitor of MEK) was added to the sciatic nerve leachate.
Neurite outgrowth analysis
Analysis of neurite outgrowth was performed as described by Fournier et al. [4] with some modifications. Briefly, the levels of neurite outgrowth in PC12 cells incubated with or without sciatic nerve leachate or 10 µM PD98059 for 3, 6, and 9 days were quantified by counting the number of neurite-bearing PC12 cells and measuring the length of the longest neurite in individual cells. Cells bearing neurites with lengths greater than twice the cell body diameter were considered positive. Differentiation efficiency was determined by calculating the ratio of positive cells to total cells. Images of three fields per well were randomly selected from wells containing at least 500 cells.
Immunofluorescence analysis
Immunofluorescence analysis was done as described previously [29] with minor modification. PC12 cells were fixed with 4% formaldehyde for 10 min at 4℃, permeabilized with 0.05% Triton X-100 for 5 min, and blocked with 5% goat serum in PBS for 30 min at room temperature. The cells were incubated with primary antibodies against β3-tubulin or microtubule-associated protein 2 (MAP2) at 4℃ overnight (1:1,000 dilutions), followed by incubation with a secondary antibody conjugated with fluorescein isothiocyanate for 60 min at 37℃. Subsequently, the cells were stained with 4′, 6-diamidino-2-phenylindol to visualize cell nuclei. Image capture was performed under a fluorescence microscope (Leica, Germany).
Western blot
Western blot analysis was performed as described previously [25]. PC12 cells were lysed by cold RIPA buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 1 mM EDTA, 1 mM Na3VO4, 1 mM NaF) with a protease inhibitor cocktail. Supernatants were collected and protein concentration determined by using Bradford protein assay reagent (Beyotime Biotechnology, China). Equal amounts of extracted protein (50 µg per lane) were denatured by heating at 95℃ for 5 min, centrifuged briefly to remove insoluble material, and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The protein was then transferred onto polyvinylidene difluoride membranes. The membranes were blocked for 1 h in 2% bovine serum albumin and then incubated overnight at 4℃ with primary antibodies against β3-tubulin, MAP2, ERK1/2, or p-ERK1/2 (1:1,000 dilutions). PBS containing 0.05% Tween 20 (PBST) was used to wash the membranes three times for 5 min each. Horseradish peroxidase-conjugated secondary antibodies were incubated for 2 h at room temperature and washed with PBST 5 times for 10 min each. Blots were developed by using ECL (Sangon Biotech, China). Densitometric software (ImageJ ver. 1.38; National Institutes of Health, USA) was used to determine the percentage distribution of blotted proteins after image acquisition by using the Gel imaging system (Aplegen, USA). The β-actin served as a control.
Statistical analysis
All data are reported as mean ± SEM values. One-way ANOVA and Duncan's multiple comparison tests were used to compare differences among groups. All calculations were performed using SPSS software (ver. 18.0; IBM, USA). Differences were considered statistically significant when p < 0.05.
Results
Sciatic nerve leachate induces neurite outgrowth of PC12 cells
The differentiation efficiencies of PC12 cells treated with cattle sciatic nerve leachate for 3, 6, and 9 days were 10.33 ± 0.67%, 32.33 ± 3.18%, and 54.67 ± 3.48%, respectively (Fig. 1). The neurite outgrowth of PC12 cells treated with cattle sciatic nerve leachate was significantly greater than that in the control group (Fig. 1). These results suggested that cattle sciatic nerve leachate can improve neurite outgrowth of rat PC12 cells.
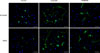
Sciatic nerve leachate enhances the expression of β3-tubulin and MAP2 protein in PC12 cells
To determine the role of cattle sciatic nerve leachate in the differentiation of rat PC12 cells into neurons, we examined the expression levels of β3-tubulin and MAP2 proteins by using immunofluorescence and Western blotting methods. The immunofluorescence assay results showed that PC12 cells in the control group only occasionally extended short processes, whereas some of the PC12 cells treated with sciatic nerve leachate for 9 days exhibited two or more neurite-like long processes, which formed a network among the cells and appeared longer and thinner than those of the cells in control group (Fig. 2). The increased expressions of β3-tubulin and MAP2 proteins in PC12 cells treated with sciatic nerve leachate can also be seen in Fig. 2. In addition, Western blot results showed that the expression levels of β3-tubulin and MAP2 proteins were increased when PC12 cells were treated with cattle sciatic nerve leachate (Fig. 3), which is in accordance with the immunofluorescence results. These results suggested that cattle sciatic nerve leachate can effectively induce neuronal differentiation of rat PC12 cells.
ERK1/2 pathway of PC12 cells was activated by sciatic nerve leachate
To identify the mechanism by which cattle sciatic nerve leachate induces neurite outgrowth, we tested the levels of p-ERK1/2 in PC12 cells. The results showed that the expression levels of p-ERK1/2 in PC12 cells treated with cattle sciatic nerve leachate for 9 days were higher by 3.99-fold than that in the control group (Fig. 4). These results suggested that the MEK/ERK1/2 pathway is activated by cattle sciatic nerve leachate.
Inhibition of ERK1/2 activation attenuates sciatic nerve leachate-induced neuronal differentiation of PC12 cells
PD98059 is widely used as a MEK 1 inhibitor. It binds to inactive MEK and prevents its activation by c-Raf, thereby hindering phosphorylation of p42/p44 MAP kinase. Thus, we investigated the effect of PD98059 on neuronal differentiation induced by cattle sciatic nerve leachate to confirm whether MEK/ERK1/2 activation is directly involved in this process. As shown in Fig. 1, the differentiation efficiencies of PC12 cells treated with 10 µM PD98059 for 3, 6, and 9 days were 3.33% ± 0.88%, 14.33% ± 1.45%, and 25% ± 2.08%, respectively. PD98059 significantly reduced sciatic nerve leachate-induced neurite outgrowth. Furthermore, the expression levels of MAP2 and β3-tubulin were reduced when cells were treated with the inhibitor PD98059 (Figs. 2 and 3). Our results suggested that MEK/ERK1/2 pathway is involved in the sciatic nerve leachate-induced neuronal differentiation of PC12 cells. In addition, we observed that neuronal differentiation of PC12 cells was only reduced by the inhibitor PD98059; it did not completely block this process, suggesting that other signaling pathways are also involved in this process.
Discussion
It is well known that neurotrophins derived from Schwann cells can activate specific cell surface receptors, which initiate a series of reactions in cells including neuronal morphology, survival, and function [13]. Some studies have revealed that it is important for Schwann cells to secrete cytokines such as nerve growth factor (NGF) [6], epidermal growth factor [17], brain-derived neurotrophic factor [15], and basic fibroblast growth factor [9] in the regeneration process of axotomized peripheral nerve fibers [20]. Moreover, there is a dramatic increase in the expression of these neurotrophins in nerve segments distal to a transection or crush injury [320]. Actually, a situation similar to nerve injury is created when the sciatic nerve is cut into fragments. Thus, many neurotrophins will be released when a sciatic nerve is cut into fragments. In accord with that theory, we successfully induced differentiation of rat adipose-derived stem cells into Schwann-like cells with sciatic nerve leachate made by cutting and soaking rat sciatic nerve in medium [12]. Although this method is effective for inducing neuronal differentiation of stem/progenitor cells, several animals have to be sacrificed to obtain enough sciatic nerve for the leaching process. Therefore, in this study, we tested whether sciatic nerve leachate derived from a different species would still have the ability to induce neuronal differentiation of rat stem/progenitor cells.
PC12 cells derived from the rat adrenal pheochromocytoma are used widely as a model of neuronal differentiation in vitro because they can respond to nerve factor treatment by showing robust neural processes and neuron-like morphology [71822]. The β3-tubulin protein is expressed most prominently in axon-like cell extensions [19], and MAP2 is mainly expressed in neuronal dendritic extensions [21]. The expression levels of β3-tubulin and MAP2 are increased when PC12 cells differentiate into neurons [1423]. In this study, we produced a leachate from cattle sciatic nerves obtained from a slaughterhouse and tested its effects on neurite outgrowth and the expressions of β3-tubulin and MAP2. The results demonstrated that cattle sciatic nerve leachate can successfully induce neuronal differentiation of rat PC12 cells. The high level of conservation of neurotrophins among species may be a reasonable explanation for these results. Although the detailed mechanism is unclear, the advantages of obtaining sciatic nerve leachate from cattle are obvious. Firstly, two 20 cm long sciatic nerves can be easily obtained from a single cow and they are sufficient for the production of 500 mL of induction medium because the sciatic nerve of cattle is long and robust. In our previous study, 14 adult rats had to be killed to produce 100 mL of sciatic nerve leachate. Secondly, sciatic nerves can be obtained free of charge when cattle are being killed in a slaughterhouse because the sciatic nerve of cattle has little or no market value. Based on our results, we speculate that sciatic nerve leachate from others animal such as rabbit, sheep, goat, pig, dog, etc., can also induce neuronal differentiation of stem/progenitor cells. Therefore, the source of material suitable for producing sciatic nerve leachate is very wide. This is beneficial for clinical applications of this method.
Lin et al. [10] reported that 15 signaling pathways are involved in the neuronal differentiation of PC12 cells and the MAPK/ERK1/2 pathway is one of them. Several lines of evidence suggest that ERK1/2, which is a part of the MAPK signaling pathway, are crucial factors in the regulation of cell survival, growth, and proliferation [52628]. In addition, previous studies revealed that MAP kinase was activated when PC12 cells were treated with NGF, and this pathway is very crucial in NGF-induced neuronal differentiation of PC12 cells [27]. Therefore, in this study, we focused on the MEK/ERK1/2 signaling cascade, one of the key signaling pathways that control neurite outgrowth [26].
Our results demonstrated that the MEK/ERK1/2 signaling pathway is involved in sciatic nerve leachate-induced neuronal differentiation of PC12 cells. However, the inhibitor PD98059 only attenuated the effect of the sciatic nerve leachate on neuronal differentiation of PC12 cells; it did not completely block this process. Thus, we speculate that other signaling pathways also contribute to sciatic nerve leachate-induced neuronal differentiation of PC12 cells. Further studies are needed to elucidate the exact molecular pathways involved in the effects of sciatic nerve leachate.
In conclusion, the presented data show that a sciatic nerve leachate obtained from cattle can effectively stimulate neuronal differentiation of rat PC12 cells through the MEK/ERK1/2 signaling pathway. Our results suggest that sciatic nerve leachates may be obtained from a variety of species and used for inducing neuronal differentiation of stem/progenitor cells. This would be particularly beneficial for clinical applications involving sciatic nerve leachate in stem cell-based treatment of nerve injury.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download