Abstract
Due to their similarities with humans in anatomy, physiology, and genetics miniature pigs are becoming an attractive model for biomedical research. We aim to establish and evaluate blood type O cells derived from Korean native pig (KNP), a typical miniature pig breed in Korea. Ten cell lines derived from 8 KNP piglets and one adult female KNP (kidney and ear tissues) were established. To confirm the presence of blood type O, genomic DNA, fucosyltransferase (FUT) expression, and immunofluorescence staining were examined. Additionally, fluorescence-activated cell sorting and somatic cell nuclear transfer were performed to investigate the normality of the cell lines and to evaluate their effectiveness in embryo development. We found no significant bands corresponding to specific blood group A, and no increase in FUT expression in cell lines derived from piglets No. 1, No. 4, No. 5, No. 8, and the adult female KNP; moreover, they showed normal levels of expression of α 1,3-galactosyltransferase and cytidine monophosphate-N-acetylneuraminic acid hydroxylase. There was no significant difference in embryo development between skin and kidney fibroblasts derived from the blood type O KNPs. In conclusion, we successfully established blood type O KNP cell lines, which may serve as a useful model in xenotransplantation research.
Pigs are useful animal models in human medical research because of their similarities to humans in physiology, anatomy, nutrition, and genetics. Compared to other animal models, pigs are economical, easily handled, and safe to work with; as well, they are associated with low ethical sensitivity because pigs are extensively used in the food industry. In particular, miniature pig breeds such as Yucatan, Hanford, and Sinclair have the advantage of similarities with human organ size and physiology, which makes miniature swine an excellent animal model for preclinical and biomedical research.
The Korean native pig (KNP) is a typical pig breed in Korea, with black hair and small body weight, and is considered to provide the most expensive and highest quality pork in Korea [4]. Due to their low productivity and poor genetic traits, compared to other commercial breeds, the number of KNPs has greatly reduced since 1986 [7]. Thus, developing an effective breeding program for maintaining or increasing KNP numbers is highly recommended. At present, there are a few reports on the use of KNPs in animal biotechnology studies, most of which are focused on its genetic traits [1112].
Somatic cell nuclear transfer (SCNT) is a powerful tool for creating viable embryos for medical, agricultural, and basic biology research. Using the SCNT technique with transgenesis methodologies is a practical approach to the production of transgenic animals, but the efficiency of producing transgenic pigs using SCNT technology is still low [1]. Previously, a swine leukocyte antigen homotype-defined KNP was successfully produced by SCNT [5], which showed that the KNP could become a valuable model for transplantation and xenotransplantation research.
In the present study, blood genotyping, fucosyltransferase (FUT) expression analysis, and fibroblast immunofluorescence staining were performed to identify blood type O piglets. In addition, fluorescence-activated cell sorting (FACS) analysis was undertaken to observe the expression of α 1,3-galactosyltransferase (alpha-Gal) in pig fibroblasts. In addition, SCNT using skin- and kidney-derived fibroblasts was performed, and cleavage and blastocyst formation rates after SCNT were evaluated.
Two KNPs from Kangwon province, Korea, were naturally mated. After 113 days, a cesarean section was performed and 8 piglets were successfully delivered. Distal parts of piglet tails, as well as kidney and ear tissues collected from the female KNP, were sampled and isolated. In order to isolate porcine fibroblasts, small pieces of tissue samples were washed thoroughly three times in 1× phosphate buffered saline (PBS), minced with micro-scissors, and washed three times with Dulbecco's modified Eagle's medium (DMEM; Invitrogen, USA) containing 10% (v/v) heat-inactivated fetal bovine serum and 1% antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin; Gibco, USA) by centrifugation at 150 × g for 2 min. The tissue pellets were suspended in washing medium, seeded into 100-mm plastic culture dishes, and cultured at 38℃ in a humidified atmosphere with 5% CO2 until grown to full confluence. Finally, they were trypsinized and cryopreserved in equal aliquots for analysis and evaluation. The entire experimental procedure was reviewed and approved by the Institutional Animal Care and Use Committee of Seoul National University in accordance with the Guide for the Care and Use of Laboratory Animals of Seoul National University (SNU-151019-4).
Total genomic DNA from the 8 newborn piglets and the female KNP were extracted from cryopreserved tissue samples by using the G-spin Total DNA Extraction Kit (iNtRON Biotechnology, Korea). The purity and integrity of the obtained DNA were assessed by using spectrophotometry (Nanodrop ND-100; Nanodrop, USA). Blood group antigen A-specific primers 5′-GCTCCCATCATCTGGGATGG-3′ and 5′-GATGTAGTAGTTGACCCTGTG-3′ were applied for antigen A detection. Conditions for polymerase chain reaction (PCR) were set at 95℃ for 10 min, then 35 cycles of 95℃ for 15 sec and 66℃ for 1 min, followed by 95℃ for 15 sec, 66℃ for 20 sec, and 95℃ for 15 sec. PCR products were electrophoresed in 1% agarose gel and stained with RedSafe (iNtRON Biotechnology) for visualization. Positive and negative control genomic DNA used in this study were derived from previous studies [1724].
According to the manufacturer's instructions, total RNA was extracted from all isolated fibroblasts by using the Easy-spin (DNA-free) Total RNA Extraction Kit (iNtRON Biotechnology). Purity and integrity of RNA were assessed by using spectrophotometry and agarose gel electrophoresis. By using the Maxime RT-PCR Premix (iNtRON Biotechnology), reverse transcription was carried out for cDNA synthesis with a total volume of 20 µL at 45℃ for 60 min. Real-time relative quantitative PCR (RQ-PCR) was conducted by using SYBR Green as the double-stranded DNA-specific fluorescent dye (Takara Bio, Japan) and the Applied Biosystems Step One Plus Real-Time PCR System (Applied Biosystems, USA). Porcine glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal standard to normalize RQ-PCR reaction efficiency and to quantify all isolated piglet-derived mRNA transcripts. RQ-PCR was performed with at least 6 replications each and using FUT1- and FUT2-specific primers on each sample independently (Table 1). The relative expression level of each mRNA transcript in the isolated cells was calculated by using the equation R = 2−(ΔCt sample – ΔCt control) as previously described [13].
For immunofluorescence analysis, all steps were performed at room temperature unless otherwise stated. Piglet-derived fibroblasts were fixed with 4% paraformaldehyde (w/v) in PBS for 30 min. Fixed fibroblasts were washed with 1× PBS and transferred into PBS containing 0.5% Triton X-100 (v/v) for 30 min to induce permeabilization. Then, they were washed three times for 5 min each with 0.05% (v/v) Tween-20 and blocked with PBS containing 2% bovine serum albumin for 1 h. After blocking nonspecific sites, piglet-derived fibroblasts were incubated with primary antibody (anti-blood group antigen A, diluted to 1:50 [Antibodies-online, UK]) for 2 h. Next, the isotype-specific secondary antibody (anti-mouse IgG coupled to Alexa Fluor 488, diluted to 1:500 [A11029; Invitrogen]) was applied for 2 h, followed by treatment with VECTASHIELD Mounting Medium with DAPI (Vector Laboratories, USA) for 10 min before observation under a microscope.
The alpha-Gal expression procedure was carried out as described previously [15]. Briefly, trypsinized piglet fibroblasts (1 × 105) from each piglet including negative control (cytidine monophosphate-N-acetylneuraminic acid hydroxylase [CMAH]- and GGTA-deleted cells [6]), positive control [24], the female KNP, piglets with blood type O (No. 1 and No. 8) and piglets with blood type A (No. 2 and No. 3) were washed in 1× PBS. Next, they were incubated with fluorescein isothiocyanate (FITC)-conjugated Bandeiraea simplicifolia isolectin B4 (BS-IB4, diluted to 1:200; Sigma-Aldrich, USA) for 1 h at 4℃. For analysis of CMAH expression, cells were incubated with purified anti-N-glycolylneuraminic acid (Neu5Gc, diluted to 1:500; BioLegend, USA) for 1 h at room temperature. Negative control cells were incubated in 1× PBS alone. Stained cells were washed twice in 1× PBS and then analyzed by using a FACSCalibur flow cytometer with CELLQUEST software (BD DIVA ver. 6.0; Becton Dickinson, USA).
Porcine ovaries were recovered from a local abattoir, placed in saline solution at 30℃–35℃, and transported to the laboratory. Follicular fluid was collected by aspiration from 3 to 6 mm follicles with an 18-gauge needle and allowed sediment to settle to the bottom of 50 mL conical tubes held at 37℃. The sediment was pooled and washed three times in washing medium comprising 9.5 g/L of tissue culture medium-199 (TCM-199; Invitrogen), 5 mM sodium hydroxide, 2 mM sodium bicarbonate, 10 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES), 0.3% polyvinyl alcohol, and 1% penicillin-streptomycin (Invitrogen). Only cumulus-oocyte complexes (COCs) with homogeneous cytoplasm and three or more layers of cumulus cells were collected and placed into in vitro maturation (IVM) medium containing TCM-199 supplemented with 2 mM sodium pyruvate, 5 µL/mL insulin transferrin selenium solution 100X (Invitrogen), 0.57 mM cysteine, 10 ng/mL epidermal growth factor, 10% porcine follicular fluid (v/v), 10 IU/mL human chorionic gonadotropin, and 10 IU/mL equine chorionic gonadotropin at 38.5℃ under 5% CO2 in 95% humidified air. After 22 h of IVM, the COCs were washed and culture was continued in hormone-free IVM medium for an additional 22 h.
To compare developmental competence between skin and kidney fibroblasts derived from blood type O KNPs, SCNT was performed as previously described [21]. Donor cells from passage numbers 4 and 5 were used. Briefly, after 44 h of IVM, COCs were denuded by gently pipetting in Tyrode's albumin lactate pyruvate (TALP) with 0.1% hyaluronidase. Denuded oocytes were stained with 5 µg/mL of bisbenzimide (Hoechst 33342) for 10 min. Then, under inverted epifluorescence microscopy, an oocyte was seized with a holding micropipette and the first polar body and nuclear material were aspirated together with a small amount of adjacent cytoplasm by using a fine glass needle in TALP medium droplets containing 7.5 µg/mL of cytochalasin B. Soon afterwards, a single cell was injected into the perivitelline space of each enucleated oocyte. For fusion, cell-oocyte couplets were gradually equilibrated with fusion medium comprising 0.28 M mannitol, 0.5 mM HEPES, and 0.1 mM MgSO4 and then fused in a 20 µL droplet of fusion medium with a single direct current (DC) pulse of 200 V/mm for 30 µsec by using an electric pulsing machine (LF101; Nepa Gene, Japan). After 30 min, fused couplets were gradually equilibrated with activation medium comprising 0.28 M mannitol, 0.5 mM HEPES, 0.1 mM CaCl2, and 0.1 mM MgSO4, after which they were placed in an activation chamber filled with activation medium, and activated with a single DC pulse of 1.5 kV/cm for 60 µsec by using a BTX ElectroCell Manipulator 2001 (BTX, USA). Electrically activated embryos were then washed and cultured in porcine zygote medium-5 (PZM-5; Funakoshi, Japan) at 38.5℃ in a humidified atmosphere with 5% O2, 5% CO2, and 90% N2 for 7 days (in vitro culture, IVC).
Cleavage and blastocyst formation rates were examined at 48 h and 168 h, respectively, during IVC. Subsequently, blastocysts from each group were collected, washed in TALP medium, and stained with 25 µg/mL of bisbenzimide in TALP medium. Stained blastocysts were placed in glycerol droplets on a glass slide, gently mounted with a coverslip, and observed under a fluorescence microscope (Nikon, Japan).
PCR bands were digitally captured with Gel Capture E-Gel 1.0.0.0 imager software. All statistical analyses including development data (cleavage and blastocyst formation rates) were performed by an unpaired t-test using GraphPad Prism 5 (GraphPad Software, USA). The experiments were repeated 5 times for each group. Results are expressed as mean ± SEM values, and all differences were considered significant at p < 0.05.
To confirm the blood genotyping of the isolated cell lines derived from the KNPs, PCR using genomic DNA, and RQ-PCR and immunofluorescence analyses were performed. The associated PCR products were represented by intense bands on RedSafe stained gels after electrophoresis. As shown in panel A in Fig. 1, significant bands corresponding to specific blood group A were found in isolated cell lines from piglets No. 2, No. 3, No. 6, and No. 7, and forming the positive control. Panels B and C in Fig. 1 shows expression of H-antigen-related genes (FUT1 and FUT2) in isolated cell lines of the piglets. The results showed that, among the groups, cell lines derived from piglets No. 2, No. 3, No. 6, and No. 7 exhibited significantly increased expression levels of FUT1. They also expressed significantly higher levels of FUT2 than the other piglets; notably high in the piglet No. 3 cell line which showed a 20-fold higher expression of FUT2 compared to that of the controls. In addition, immunofluorescence staining showed a similar pattern of blood antigen A under fluorescence microscopy for the cell lines derived from piglets No. 2, No. 3, No. 6, and No. 7 (Fig. 2). These results confirmed that cell lines derived from piglets No. 1, No. 4, No. 5, No. 8, and the female KNP were of blood type O.
The expression of alpha-Gal was examined by using trypsinized porcine fibroblasts. Seven generated cell lines, including a negative control (CMAH- and GGTA-deleted cells), a positive control (wild-type cells), blood type O cells (piglets No. 1 and No. 8 and the female KNP), and blood type A cells (piglets No. 2 and No. 3), were evaluated. Relative protein quantification by FACS analysis confirmed the positive expression of alpha-Gal in all cell lines (panel A in Fig. 3). Moreover, with the same samples, the expression of CMAH, a representative non-Gal antigen, was analyzed by using Anti-Neu5Gc (panel B in Fig. 3). As shown in the results, the CMAH expression level was similar to alpha-Gal expression in all cell types.
To examine the ability of donor cells used for SCNT to support in vitro development, cleavage and blastocyst formation rates were compared between kidney cells and skin fibroblasts derived from blood type O KNPs (Table 2). A total of 227 and 228 embryos were produced by using skin fibroblast and kidney donor cells, respectively. As shown in Table 2, there were no significant differences in cleavage, blastocyst formation rates, and total blastocyst cell numbers (86.78 ± 3.84, 31.28 ± 5.95, and 45.43 ± 5.17 vs. 87.72 ± 2.17, 28.07 ± 2.99, and 45.29 ± 5.39, respectively) between the two donor cell types.
It is well known that SCNT is a valuable tool for generating cloned embryos for various biomedical research studies. In particular, using established SCNT and stem cell technology has proven to be of great value for modeling clinical applications of xenotransplantation, by making it possible to bulk-produce immunosuppressive organs [8]. Due to an insufficient supply of human organs to support the needs of patients in terminal organ failure, it is widely understood that an alternative source of organs would be invaluable. Because of their comparable size and physiology to that of humans, as well as their breeding characteristics and various ethical considerations, pigs have been considered the best source for xenotransplantation studies and a potential source of transplantable organs [310]. These features are especially true for the miniature pig, which has a suitable body mass and is easy to handle. These characteristics make miniature pigs a potentially useful model for assorted research studies. Recently, although several miniature pigs have been successfully cloned via SCNT [92326], the cloning efficiency was notably low, which has been an obstacle to the promising application of miniature pigs in biomedical research. Donor cell type is one of the more important factors that influence the outcomes of SCNT, therefore, cell types should be well established and evaluated before practical use.
Recent reports have assessed the ABO blood groups of pigs by using various methods and have indicated that it is difficult to identify blood phenotypes in young piglets [20]. Generally, pigs do not display blood type B, but a blood type A substance is present on the surface of pig red blood cells (RBCs). It is well understood that ABO phenotyping is a crucial factor influencing allotransplantation because mismatching of the AO blood group antigens between donor and recipient can cause substantial adverse effects on graft survival and can reduce the success rate of graft transplantation in both humans [19] and pigs [14]. In xenotransplantation studies, several trials have been undertaken between species, such as pig-to-primate transplants. However, in many transplant studies using pigs, blood group antigens remained an obscure or confounding variable [20]. Because RBCs and vascular surfaces of the blood type O pig only present the alpha-Gal antigen without any presence of other AB antigens [20], using cells and tissues of the blood type O group would reduce xenograft rejection reactions and make the pig a more useful model for xenotransplantation research.
In the present study, we successfully established blood type O KNP cell lines that normally express CMAH and alpha-Gal. Among the isolated cell lines, blood O genotyping was confirmed in cell lines derived from KNP piglets No. 1, No. 4, No. 5, No. 8, and a female KNP. This was confirmed by using PCR to show the absence of significant bands corresponding to specific blood group A and the lack of expression of FUT1 and FUT2 genes, as well as the absence of specific blood type A antigen staining under fluorescence microscopy. The FUT gene family encodes a group of proteins shown to be essential for normal biological functions. FUT1 encodes α1, while 2-FUT supports the presence of H antigen, the precursor of A and B antigens, on RBCs. On the other hand, FUT2 encodes α-FUT s which determine H expression in secretions [25]. In the absence of these enzymes, no H antigens are produced. Therefore, determining the expressions of FUT1 and FUT2 genes by RQ-PCR, which are directly connected with H antigen production, could be used as a tool to categorize blood genotypes in pigs. To investigate the normality of the isolated porcine cell lines, we also examined the expression of alpha-Gal and CMAH by performing FACS analysis. The alpha-Gal epitope (Galalpha1-3Galbeta1-(3)4GlcNAc-R) is a common carbohydrate structure that is absent in humans and non-human primates but is naturally presented on glycolipids and glycoproteins in most mammals, including pigs. It has a wide distribution on various pig tissues especially on vascular epithelial cells [216]. The presence of the alpha-Gal epitope causes xenograft rejection, and many research groups have tried, by breeding pigs that do not express Gal, to overcome the rejection reaction by knocking out the gene for the enzyme alpha-Gal [1018]. Not only the alpha-Gal epitope, but also CMAH which mediates Neu5Gc another well-known xenoantigen expressed by RBCs, have been studied in an attempt to make possible a clinical application of pig RBCs for human transfusion [22]. Neu5Gc is a non-Gal antigen target, only present on human erythrocytes, that is recognized by human antibodies. In our results, all cell lines positively expressed alpha-Gal and CMAH including blood type O cell lines (KNP piglets No. 1, No. 4, No. 5, No. 8, and the female KNP). Therefore, these cell lines could be prospectively used in biological research, especially in xenotransplantation. Moreover, to compare the efficiency of blood type O cell lines as donor cells for in vitro embryo development through porcine SCNT, skin fibroblasts and kidney cells derived from the blood type O female KNP were examined. As shown by the results, there were no significant differences in cleavage, blastocyst formation rates, and total blastocyst cell numbers between the two donor cell types.
In conclusion, we successfully established blood type O KNP cell lines. The blood genotyping was confirmed by several molecular procedures, and the normal expression of alpha-Gal and CMAH in these cell lines was confirmed. We suggest that these blood type O cell lines may be useful sources of donor cell nuclei for the SCNT technique and beneficial in the generation of valuable porcine models for xenotransplantation research.
Figures and Tables
Fig. 1
Polymerase chain reaction (PCR) and real-time relative quantitative PCR results for isolated cell lines from 8 Korean native pig (KNP) piglets (No. 1–8) and skin fibroblasts of one female KNP. (A) PCR amplification products of the isolated cell lines subjected to agarose gel electrophoresis. Size of specific blood type A PCR products are approximately 340 bp. Comparison of mRNA expression levels (mean ± SEM) of FUT1 (B) and FUT2 (C). Within the same mRNA transcript, bars with asterisks indicate significantly high expression (p < 0.05). The experiment was replicated 3 times. (+), positive control; (−), negative control; M, marker; S, skin fibroblasts from female KNP; 1–8, cell lines derived from piglets No. 1–8, respectively.
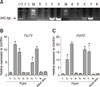
Fig. 2
Immunofluorescence staining of anti-blood group antigen A in isolated cell lines from 8 Korean native pig (KNP) piglets (No. 1–8) and skin fibroblasts of one female KNP. In each sample, bright field and immunofluorescence staining under fluorescence microscopy were performed. Scale bars = 100 µm.

Fig. 3
Relative glycol-protein quantification obtained by fluorescence-activated cell sorting analysis of α 1,3-galactosyltransferase (alpha-Gal; A) and cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH; B) in isolated cell lines including skin fibroblasts of female Korean native pig (KNP), blood type O cells from piglets No. 1 and No. 8, and blood type A cells from piglets No. 2 and No. 3. (+), positive control (wild-type cells); (−), negative control (CMAH- and GGTA-deleted cells); 1, cell lines derived from piglet No. 1; 8, cell lines derived from piglet No. 8; S, skin fibroblasts derived from the female KNP; 2, cell lines derived from piglet No. 2; 3, cell lines derived from piglet No. 3.

Acknowledgments
This study was supported by the Korea National Research Foundation (No. 2016M3A9B6903410, 2015R1C1A2A01054373) as well as the Research Institute for Veterinary Science and BK21 PLUS Program.
References
1. Cho SK, Hwang KC, Choi YJ, Bui HT, Nguyen VT, Park C, Kim JH, Kim JH. Production of transgenic pigs harboring the human erythropoietin (hEPO) gene using somatic cell nuclear transfer. J Reprod Dev. 2009; 55:128–136.

2. Dor FJ, Cheng J, Alt A, Cooper DK, Schuurman HJ. Galα1,3Gal expression on porcine pancreatic islets, testis, spleen, and thymus. Xenotransplantation. 2004; 11:101–106.

3. He J, Li Q, Fang S, Guo Y, Liu T, Ye J, Yu Z, Zhang R, Zhao Y, Hu X, Bai X, Chen X, Li N. PKD1 mono-allelic knockout is sufficient to trigger renal cystogenesis in a mini-pig model. Int J Biol Sci. 2015; 11:361–369.

4. Hur SJ, Jeong TC, Kim GD, Jeong JY, Cho IC, Lim HT, Kim BW, Joo ST. Comparison of live performance and meat quality parameter of cross bred (Korean native black pig and landrace) pigs with different coat colors. Asian-Australas J Anim Sci. 2013; 26:1047–1053.

5. Hwang IS, Kwon DJ, Oh KB, Ock SA, Chung HJ, Cho IC, Lee JW, Im GS, Hwang S. Production of cloned Korean native pig by somatic cell nuclear transfer. Dev Reprod. 2015; 19:79–84.

6. Kim GA, Lee EM, Jin JX, Lee S, Taweechaipaisankul A, Hwang JI, Alam Z, Ahn C, Lee BC. Generation of CMAHKO/GTKO/shTNFRI-Fc/HO-1 quadruple gene modified pigs. Transgenic Res. 2017; 26:435–445.

7. Kim KS, Yeo JS, Kim JW. Assessment of genetic diversity of Korean native pig (Sus scrofa) using AFLP markers. Genes Genet Syst. 2002; 77:361–368.

8. Kim S, Kim JH, Lee E, Jeong YW, Hossein MS, Park SM, Park SW, Lee JY, Jeong YI, Kim HS, Kim YW, Hyun SH, Hwang WS. Establishment and characterization of embryonic stem-like cells from porcine somatic cell nuclear transfer blastocysts. Zygote. 2010; 18:93–101.

9. Kurome M, Ishikawa T, Tomii R, Ueno S, Shimada A, Yazawa H, Nagashima H. Production of transgenic and non-transgenic clones in miniature pigs by somatic cell nuclear transfer. J Reprod Dev. 2008; 54:156–163.

10. Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, Murphy CN, Carter DB, Hawley RJ, Prather RS. Production of α-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002; 295:1089–1092.

11. Lee JB, Jung EJ, Park HB, Jin S, Seo DW, Ko MS, Cho IC, Lee JH, Lim HT. Genome-wide association analysis to identify SNP markers affecting teat numbers in an F2 intercross population between Landrace and Korean native pigs. Mol Biol Rep. 2014; 41:7167–7173.

12. Lee KT, Lee YM, Alam M, Choi BH, Park MR, Kim KS, Kim TH, Kim JJ. A whole genome association study on meat quality traits using high density SNP chips in a cross between Korean native pig and Landrace. Asian-Australas J Anim Sci. 2012; 25:1529–1539.

13. Lee S, Park EJ, Moon JH, Kim SJ, Song K, Lee BC. Sequential treatment with resveratrol-trolox improves development of porcine embryos derived from parthenogenetic activation and somatic cell nuclear transfer. Theriogenology. 2015; 84:145–154.

14. Leight GS, Kirkman R, Rasmusen BA, Rosenberg SA, Sachs DH, Terrill R, Williams GM. Transplantation in miniature swine. III: Effects of MSLA and A-O blood group matching on skin allograft survival. Tissue Antigens. 1978; 12:65–74.

15. Lutz AJ, Li P, Estrada JL, Sidner RA, Chihara RK, Downey SM, Burlak C, Wang ZY, Reyes LM, Ivary B, Yin F, Blankenship RL, Paris LL, Tector AJ. Double knockout pigs deficient in N-glycolylneuraminic acid and Galactose α-1,3-Galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013; 20:27–35.

16. McKenzie IFC, Xing PX, Vaughan HA, Prenzoska J, Dabkowski PL, Sandrin MS. Distribution of the major xenoantigen (gal(α1-3)gal) for pig to human xenografts. Transpl Immunol. 1994; 2:81–86.

17. Park SJ, Cho B, Koo OJ, Kim H, Kang JT, Hurh S, Kim SJ, Yeom HJ, Moon J, Lee EM, Choi JY, Hong JH, Jang G, Hwang JI, Yang J, Lee BC, Ahn C. Production and characterization of soluble human TNFRI-Fc and human HO-1(HMOX1) transgenic pigs by using the F2A peptide. Transgenic Res. 2014; 23:407–419.

18. Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, Jobst PM, Sharma SB, Lamborn AE, Garst AS, Moore M, Demetris AJ, Rudert WA, Bottino R, Bertera S, Trucco M, Starzl TE, Dai Y, Ayares DL. Production of α1,3-galactosyltransferase-deficient pigs. Science. 2003; 299:411–414.

20. Smith DM, Newhouse M, Naziruddin B, Kresie L. Blood groups and transfusions in pigs. Xenotransplantation. 2006; 13:186–194.

21. Taweechaipaisankul A, Jin JX, Lee S, Kim GA, Lee BC. The effects of canthaxanthin on porcine oocyte maturation and embryo development in vitro after parthenogenetic activation and somatic cell nuclear transfer. Reprod Domest Anim. 2016; 51:870–876.

22. Wang ZY, Burlak C, Estrada JL, Li P, Tector MF, Tector AJ. Erythrocytes from GGTA1/CMAH knockout pigs: implications for xenotransfusion and testing in non-human primates. Xenotransplantation. 2014; 21:376–384.

23. Wei H, Qing Y, Pan W, Zhao H, Li H, Cheng W, Zhao L, Xu C, Li H, Li S, Ye L, Wei T, Li X, Fu G, Li W, Xin J, Zeng Y. Comparison of the efficiency of Banna miniature inbred pig somatic cell nuclear transfer among different donor cells. PLoS One. 2013; 8:e57728.
24. Yeom HJ, Koo OJ, Yang J, Cho B, Hwang JI, Park SJ, Hurh S, Kim H, Lee EM, Ro H, Kang JT, Kim SJ, Won JK, O'Connell PJ, Kim H, Surh CD, Lee BC, Ahn C. Generation and characterization of human heme oxygenase-1 transgenic pigs. PLoS One. 2012; 7:e46646.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download