Abstract
Streptococcus dysgalactiae subspecies equisimilis (SDSE) is an emerging pathogen in animals and humans. Herein, we describe two clinical swine cases of SDSE infection presenting with lameness, neurological signs, or sudden death. Pathological examination indicated suppurative arthritis, encephalitis, and multifocal abscesses in kidney and heart. The β-hemolytic colonies obtained from joint samples of each case were identified as SDSE. The two isolates had low minimum inhibitory concentrations for β-lactams, and they presented the same virulence gene profile (slo−/sagA+/pSTKP8+). Molecular analysis by multilocus sequence typing identified the SDSE isolates from cases 1 and 2 as sequence types 315 and 252, respectively.
Streptococcus dysgalactiae subspecies equisimilis (SDSE) is a β-hemolytic Streptococcus belonging to Lancefield groups A, C, G, or L [38]. SDSE has been recovered from nasal and throat secretions, tonsils, and vaginal and preputial secretions of swine [3]. In piglets, the most common sources of infection are vaginal secretions and milk from postparturient sows, and the major routes of transmission are traumatic injuries to the feet and skin lesions resulting from the rough floor surfaces of farrowing pens [316]. SDSE infections in pigs can result in arthritis, endocarditis, or meningitis, creating a loss of productivity in the pig industry. In humans, the incidence of SDSE infections, which can result in septic arthritis, pneumonia, endocarditis, or meningitis, is increasing worldwide [915]. Although numerous studies have reported that SDSE of animal origin is clearly distinct from that in humans, two recent studies have suggested that SDSE-associated human streptococcosis may be a zoonotic infection [81113]. Herein, we describe two clinical cases of piglets with SDSE infection and determine the minimum inhibitory concentration (MIC) as well as the virulence gene profile of the isolates. Furthermore, multilocus sequence typing (MLST) analysis was performed to elucidate the molecular characteristics of the isolates and to evaluate the clinical relevance of genetic relatedness between strains recovered from humans and pigs.
In case 1, four 10-day-old piglets showing lameness and neurological signs were submitted to the Animal and Plant Quarantine Agency (APQA) for disease diagnosis on May 2013. Grossly, the carpal and tarsal joints were enlarged due to yellowish synovial fluids and nodules in three piglets (panel A in Fig. 1), and yellowish fragile materials were attached to the epicardium and pericardium in one piglet (panel B in Fig. 1). In case 2, three neonatal piglets, showing lameness due to abrasion injuries on the feet and legs, from a farm with relatively high piglet mortality rates, were submitted to the APQA for diagnosis on August 2015. Notably, veterinarians found blood-stained cement residue remaining on the floor of the pens in which the piglets had been kept prior to their sudden death (panels A and B in Fig. 2). Grossly, yellowish discoloration with turbid materials and redness were found in the femoral synovial cavity of two piglets (panel C in Fig. 2). In addition, moderate to severe periarticular fibrous proliferation was observed. Further, inguinal lymph node enlargement and serous atrophy of fat were observed in two piglets.
After necropsy, tissue samples from major organs were fixed in 10% neutral-buffered formalin and embedded in paraffin wax. The embedded tissues were sectioned at a 4-µm thickness, and then underwent hematoxylin and eosin and Gram staining. In case 1, the histopathological lesions were arthritis and suppurative encephalitis with multiple microabscesses in three piglets. Those microabscesses consisted of abundant polymorphonuclear cells (panel C in Fig. 1). Multiple abscesses with intralesional bacteria were observed in the kidney (panel D in Fig. 1) and epicardium (panels E and F in Fig. 1) in one piglet. In case 2, no specific histopathologic lesions were observed, except for suppurative arthritis.
Bacterial cultures from carpal, tarsal, and knee joint, meninges, and epicardium samples from case 1, as well as femoral joint samples from case 2, were performed. Samples were cultured on 5% sheep blood agar (Asan Pharm., Korea) under 5% CO2 at 37℃ for 24 h. The β-hemolytic colonies were obtained from the carpal, tarsal, and knee joints and meninges of four piglets, as well as from the epicardium of one piglet in case 1 and the right femoral joint of one piglet in case 2. To identify the isolates, 16S ribosomal RNA gene sequencing was performed by using an ABI Prism BigDye Terminator Cycler Sequencing Ready Reaction Kit (Applied Biosystems, USA). Sequences obtained from cases 1 and 2 were compared with sequences in public sequence databases (National Center for Biotechnology Information, USA); all of the isolates presented 99.9% similarity to the SDSE reference strain (GenBank accession No. CP002215). To identify the Lancefield serological group, a Strep LA grouping kit (Denka Seiken, Japan) was used. Isolates from both cases were categorized into Lancefield group C; this result is consistent with that of a previous study reporting the classification of SDSE isolated from a pig with systemic suppurative inflammation into group C [5]. In both cases in the present study, other pathogenic bacteria causing systemic infection, such as Haemophilus parasuis or Streptococcus suis, were not isolated. The samples were additionally negative for all tested viruses, including classic swine fever virus, porcine circovirus type 2, and porcine reproductive and respiratory syndrome virus. The results suggest that veterinarians should consider SDSE as a potential causative agent in piglets presenting with severe arthritis or sudden death; particularly, when abrasion of feet, due to a coarse floor surface as found in case 2, is additionally observed. The observations in the present study highlight the importance of management of floor surfaces in farrowing pens because coarse flooring is a risk factor for SDSE infection.
Antimicrobial therapy is widely used to treat streptococcosis in pigs and humans [39]. In this study, MICs were determined via a broth microdilution system (Sensititre System; TREK Diagnostic Systems, UK) with 96-well antimicrobial testing plates (BOPO6F; TREK Diagnostic Systems) in accordance with the manufacturer's instructions and Clinical and Laboratory Standards Institute guidelines [2]. Streptococcus pneumoniae ATCC 49619 was used as the quality control strain. Two SDSE isolates from the tarsal joint sample in case 1 and femoral joint sample of case 2 were found to have the same MIC values for all antibiotics except gentamicin (Table 1). Notably, low MIC values were observed for the β-lactams tested; this is consistent with the results of previous studies in pigs and humans worldwide [56915]. These results suggest the importance of β-lactam agents for the treatment of SDSE infection in pigs. Meanwhile, some human-derived SDSE strains that are resistant to β-lactams require treatment with cell-penetrating antimicrobials, preferably macrolides, or, in the case of macrolide-resistant strain, tetracyclines, fluoroquinolones, or clindamycin [10]. However, the swine-derived isolates in the present work, as well as in previous studies, showed relatively high MIC values and resistance to macrolides, tetracyclines, and clindamycin [56]. Given that SDSE isolates from animals are known to have a potential zoonotic risk, the antimicrobial resistance observed in the present swine-derived strains warns of the possibility of similar treatment failure in human SDSE infection. Therefore, the current results, which are of potential public health significance, indicate that it is necessary to establish antimicrobial stewardship guidelines for SDSE infection, both in human and veterinary medicine.
In the present study, three non-superantigenic virulence genes were screened by polymerase chain reaction targeting streptolysin O (slo), streptolysin S (sagA), and streptokinase (pSTKP8) in two isolates from the tarsal joint sample in case 1 and femoral sample of case 2. The primer sequences were as described in previous studies, and amplification was carried out under the following conditions [14]: 30 cycles of 94℃ for 1 min, 55℃ for 1 min, and 72℃ for 1 min. The virulence gene profiles of isolates from both cases was slo−/sagA+/pSTKP8+ (Table 1), which is consistent with the results in a previous report investigating SDSE strains from Yorkshire pigs in Japan [5]. However, the result was inconsistent with that of a previous study of human-derived SDSE (slo+/sagA+/pSTKP8+), implying that isolates of swine origin may possess different virulence gene profiles from those recovered from humans [514]. A recent study suggested that slo represents a cytotoxic factor that likely contributes to SDSE-mediated necrotizing soft tissue infections [12]. This may represent a plausible explanation for the observed discrepancy in virulence gene profiles between humans and pigs.
To elucidate their molecular epidemiology, the two isolates from the joint samples of each case were characterized by using the MLST scheme for SDSE provided by the public MLST databases (pubMLST, UK), a scheme that is based on internal fragments of seven housekeeping genes gki, gtr, murI, mutS, recP, xpt, and atoB. Unique sequences at each locus were assigned allele numbers, and a combination of seven allele numbers for each isolate was used to define its sequence type (ST). Isolates from cases 1 and 2 were identified as a new ST and as ST252, respectively. The novel ST of the case 1 isolate was submitted to the pubMLST and assigned as ST315 (Table 1). PHYLOViZ v2.0 [7] was used to establish a minimum spanning tree, which represents the relationships among STs from the pubMLST (Fig. 3). Based on the MLST database, the isolate from case 1 (ST315) was identified as a triple-locus variant (TLV) of a ST234 horse-derived strain from the United States. The ST of case 2 (ST252) had previously been identified in pigs with peritonitis in Germany. Interestingly, ST252 was a single-locus variant (SLV) of ST253 and ST255 derived from pigs in Sweden and humans in Portugal, respectively. Additionally, ST252 was a TLV of ST254 derived from dogs in the United Kingdom. Given that one of the SDSE isolate in this study was linked at the SLV level with that isolated from human, the result supports the hypothesis that this bacterium may have a zoonotic possibility [1113]. Nevertheless, future studies should aim to perform molecular characterization of large numbers of SDSE isolates from pigs to gain a better understanding of the SDSE transmission dynamics, zoonotic possibilities, and microevolutionary events.
Figures and Tables
Fig. 1
Case 1. Gross and histopathological findings. (A) Piglet. Note the enlarged tarsal joint (arrow). (B) Thoracic cavity. Yellowish materials (asterisks) were attached to the epicardium. (C) Cerebrum. Suppurative encephalitis with multifocal microabscesses (circle) was observed. Hematoxylin and eosin (H&E) stain. 400×. (D) Kidney. Note the multifocal microabscesses (circle) with intralesional bacteria (arrow). H&E stain. 400×. (E) Heart. Note the multifocal microabscesses with intralesional bacteria (arrow). H&E stain. 400×. (F) Heart. Gram-positive cocci were observed in the myocardium. Gram stain. 1,000×. Scale bars = 20 µm (C–F).
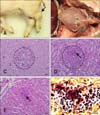
Fig. 2
Case 2. Farm floor and gross findings. (A) Concrete residue on the pen floor. (B) Note the blood-stained concrete. (C) Hip joint of piglet. Note the yellowish fragile materials and redness of synovial cavity with periarticular thickening (arrows).

Fig. 3
A goeBURST diagram for MLST data of Streptococcus dysgalactiae subspecies equisimilis isolates. Each circle in the diagram represents a sequence type (ST), with the size of the circle and its colored segments proportional to the number and origin of isolates, respectively. Numbers on branches represent the number of loci different from that of the founder ST. Isolates from case 1 (ST315) and case 2 (ST252) in this study are indicated by dashed arrow and arrow, respectively.

Table 1
Minimum inhibitory concentration (MIC) values, virulence genes, and multilocus sequence typing data for Streptococcus dysgalactiae subspecies equisimilis isolates from piglets

TIO, ceftiofur; PEN, penicillin; AMP, ampicillin; CTE, chlortetracycline; OTE, oxytetracycline; DAN, danofloxacin; ENR, enrofloxacin; FFC, florfenicol; GEN, gentamicin; NEO, neomycin; SDM, sulphadimethoxine; SXT, trimethoprim/sulfamethoxazole; CLI, clindamycin; TYL, tylosin; TIL, tilmicosin; TUL, tulathromycin; SPT, spectinomycin; TIA, tiamulin. *Allelic profile, gki-gtr-murI-mutS-recP-xpt-atoB. †ST, sequence type.
Acknowledgments
This work was supported by the Animal and Plant Quarantine Agency (B-1543069-2017-18) and Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (114058-03) through the Agri-Bio Industry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs of Korea.
References
1. Caballero AR, Lottenberg R, Johnston KH. Cloning, expression, sequence analysis, and characterization of streptokinases secreted by porcine and equine isolates of Streptococcus equisimilis. Infect Immun. 1999; 67:6478–6486.

2. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals. Approved Standard-Fourth Edition. Wayne: CLSI;2013. CLSI document VET01-A4.
3. Gottschalk M. Streptococcosis. In : Straw BE, Zimmerman JJ, D'Allaire S, Taylor DJ, editors. Diseases of Swine. 10th ed. Ames: Blackwell Publishing Professional;2012. p. 841–855.
4. Ikebe T, Murayama S, Saitoh K, Yamai S, Suzuki R, Isobe J, Tanaka D, Katsukawa C, Tamaru A, Katayama A, Fujinaga Y, Hoashi K, Watanabe H. The Working Group for Streptococci in Japan. Surveillance of severe invasive group-G streptococcal infections and molecular typing of the isolates in Japan. Epidemiol Infect. 2004; 132:145–149.

5. Kasuya K, Yoshida E, Harada R, Hasegawa M, Osaka H, Kato M, Shibahara T. Systemic Streptococcus dysgalactiae subspecies equisimilis infection in a Yorkshire pig with severe disseminated suppurative meningoencephalomyelitis. J Vet Med Sci. 2014; 76:715–718.

6. Moreno LZ, da Costa BL, Matajira CE, Gomes VT, Mesquita RE, Silva AP, Moreno AM. Molecular and antimicrobial susceptibility profiling of Streptococcus dysgalactiae isolated from swine. Diagn Microbiol Infect Dis. 2016; 86:178–180.

7. Nascimento M, Sousa A, Ramirez M, Francisco AP, Carriço JA, Vaz C. PHYLOViZ 2.0: providing scalable data integration and visualization for multiple phylogenetic inference methods. Bioinformatics. 2017; 33:128–129.

8. Pinho MD, Erol E, Ribeiro-Gonçalves B, Mendes CI, Carriço JA, Matos SC, Preziuso S, Luebke-Becker A, Wieler LH, Melo-Cristino J, Ramirez M. Beta-hemolytic Streptococcus dysgalactiae strains isolated from horses are a genetically distinct population within the Streptococcus dysgalactiae taxon. Sci Rep. 2016; 6:31736.

9. Rantala S. Streptococcus dysgalactiae subsp. equisimilis bacteremia: an emerging infection. Eur J Clin Microbiol Infect Dis. 2014; 33:1303–1310.

10. Savini V, Catavitello C, Talia M, Manna A, Pompetti F, Di Bonaventura G, Di Giuseppe N, Febbo F, Balbinot A, Di Zacomo S, Esattore F, D'Antonio D. Beta-lactam failure in treatment of two group G Streptococcus dysgalactiae subsp. equisimilis pharyngitis patients. J Clin Microbiol. 2008; 46:814–816.

11. Schrieber L, Towers R, Muscatello G, Speare R. Transmission of Streptococcus dysgalactiae subsp. equisimilis between child and dog in an Aboriginal Australian community. Zoonoses Public Health. 2014; 61:145–148.

12. Siemens N, Kittang BR, Chakrakodi B, Oppegaard O, Johansson L, Bruun T, Mylvaganam H. INFECT Study Group. Svensson M, Skrede S, Norrby-Teglund A. Increased cytotoxicity and streptolysin O activity in group G streptococcal strains causing invasive tissue infections. Sci Rep. 2015; 5:16945.

13. Silva LG, Genteluci GL, Corrêa de Mattos M, Glatthardt T, Sá Figueiredo AM, Ferreira-Carvalho BT. Group C Streptococcus dysgalactiae subsp. equisimilis in south-east Brazil: genetic diversity, resistance profile and the first report of human and equine isolates belonging to the same multilocus sequence typing lineage. J Med Microbiol. 2015; 64:551–558.

14. Sunaoshi KS, Aburahashi H, Kobayashi R, Yamamoto Y, Okuzumi K, Yoshida A, Misawa Y, Adachi K, Ubukata K. [Emm typing by genetic identification of Streptococcus dysgalactiae subsp. equisimilis and susceptibility to oral antibiotics]. Kansenshogaku Zasshi. 2006; 80:488–495. Japanese.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download