Abstract
Liver cancer occurs very frequently worldwide and hepatocellular carcinoma (HCC) accounts for more than 80% of total primary liver cancer cases. In this study, the anticarcinogenic effects of resveratrol against hepatitis B virus (HBV)-induced HCC were investigated by using HBV X-protein-overexpressing Huh7 (Huh7-HBx) human hepatoma cells. MTT assay showed that resveratrol decreased cell viability. Fluorescence-activated cell-sorter analysis showed that resveratrol induced G1 cell cycle arrest without increasing the sub-G1 phase cell population. Therefore, we evaluated its effect on regulation of cyclin D1, which is critically involved in G1/S transition. Resveratrol lowered cyclin D1 transcription. Western blot analysis of the effects of resveratrol on upstream cyclin D1 transcriptional signaling, extracellular signal-related kinase (ERK), p90RSK, Akt, and p70S6K revealed inhibition of Akt but not the ERK signaling pathway. Collectively, the results indicate that resveratrol inhibits Huh7-HBx proliferation by decreasing cyclin D1 expression through blockade of Akt signaling. We investigated the anticarcinogenic effect and mechanism of resveratrol in xenograft model mice implanted with Huh7-HBx cells. Intraperitoneal resveratrol injection reduced tumor size in the mice. Expression of survivin was reduced, but cyclin D1 was not affected. The results demonstrate that resveratrol treatment may help manage HBV-induced HCC by regulating survivin.
Hepatocellular carcinoma (HCC), the most widespread and malignant cancer globally, occurs extensively in Asia as well as in central and west Africa [15]. Currently, HCC has the sixth highest incidence of all cancers, worldwide, and numerous new patients are diagnosed every year. Furthermore, HCC is estimated to have the third highest annual cancer mortality rate [23]. Pre-existing hepatitis B virus (HBV) infection is the main etiological factor for HCC [15]. Despite the increasing availability and administration of HBV vaccinations worldwide, HBV remains the main cause of development of HCC [30], and outbreaks of HBV-induced HCC are expected to increase regularly [15]. Recent studies have indicated that the interaction of viral and host genetic factors may be critically involved in both the sensitivity to HBV and the development of disease [29]. In addition, it has been reported that the relationship between HBV infection and the progression of HCC may mainly involve viral factors. Recent studies have reported that HB viral loads are related to malignant transformation of primary hepatocytes [3435]. In 1975, the close link between chronic HBV infection and the progression of HCC was first reported [4], and the anti-apoptotic activity of the HBV X protein (HBx) [728] has been recognized as the predominant cause of this tumor. The molecular mechanisms underlying the carcinogenicity of HBV involve transactivation by and abnormal accumulation of viral proteins, gene activation, and recombination of the HBV subgenome [36]. However, the precise mechanisms of HBV-induced carcinogenesis have not been fully elucidated.
HBx, a multi-functional viral factor associated with HCC, has been reported to participate in the life cycle of the virus and in the development of HCC. These activities are mediated by signaling cascades that participate in the modulation of cell proliferation and survival including the STAT3/survivin signaling pathway. HBx promotes apoptosis of p53/p73-expressing hepatoma cells [16]. The complex, cell-context-dependent interaction between HBx and p53 tumor suppressor family members in the modulation of apoptosis is crucial for induction of HBV-associated HCC and in anticancer therapy [16].
Resveratrol (3,5,4′-trihydroxystilbene) is a polyphenolic antioxidant compound produced by plants and is reported to be present in berries, grapes, and peanuts [32]. It has also been observed in Japanese knotweed [33]. Resveratrol has been reported to inhibit the STAT3/survivin signaling pathway, which blocks apoptosis of tumor cells [25]. STAT proteins are involved in cell proliferation and apoptosis and act as cytokine signaling molecules and downstream effectors of growth factor receptors [11]. A recent study showed that the promoter of tumor suppressor genes is methylated by acetylated STAT3, inhibiting gene transcription. Additionally, it has been shown that resveratrol induces promoter demethylation and inhibits STAT3 acetylation, restoring the expression of tumor suppressor genes [19]. The STAT3 family and survivin, its downstream effector, are involved in the regulation of cell survival and proliferation, and survivin normally has a role as anti-apoptotic protein [25]. The associated Akt signaling pathway is normally decreased in apoptosis, and cyclin is involved in the regulation of cell cycle progression [22]. However, the effect of resveratrol on HBx-associated HCC development and its molecular mechanisms have not been elucidated.
Therefore, in the present study, we investigated the effect of resveratrol on HBx-associated HCC proliferation and the underlying molecular mechanisms by using HBx-overexpressing Huh7 (Huh7-HBx) human hepatoma cells. Moreover, we investigated resveratrol's effects on expression of survivin, downstream effectors of the STAT3 pathway, and cyclin D1 in BALB/c nude mice implanted with Huh7-HBx cells.
Resveratrol, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) powder, and propidium iodide (PI) were purchased from Sigma-Aldrich (USA). RNase was purchased from Amresco (USA). The following antibodies against target proteins were purchased from the specified manufacturers. HBx, survivin, phosphorylated (p)-p70S6K, and p70S6K (Cell Signaling Biotechnology, USA), p-ERK, ERK, and cyclin D1 (Santa Cruz Biotechnology, USA), p-p90RSK and p90RSK (Signal Way Antibody, USA), p-Akt, Akt, p-p53, and p-53 (Pierce Chemical, USA), and β-actin (Sigma-Aldrich). Horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology. Dulbecco's modified Eagle's medium (DMEM), Roswell Park Memorial Institute (RPMI) 1640 medium, fetal bovine serum (FBS), and penicillin/streptomycin were purchased from Gibco (USA).
Cytomegalovirus (CMV)-expressing Huh7 and Huh7-HBx human hepatoma cells were provided by Dr. Guhung Jung (College of Natural Science, Seoul National University, Korea). The cells were cultured in DMEM containing penicillin (100 U/mL), streptomycin (100 µg/mL), and 10% FBS at 37℃ under an atmosphere of 5% CO2.
The Huh7 human hepatoma cells were seeded into 6-cm dishes at a density of 1.5 × 105 cells per dish and cultured in RPMI 1640 medium supplemented with 10% FBS before resveratrol treatment. The cells were then treated with resveratrol at varying concentrations, after which the cellular proteins were harvested and centrifuged at 18,500 × g at 4℃ for 10 min. The supernatants were collected and the proteins were separated by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto nitrocellulose membranes (Pall, USA). The membranes were blocked with TBS containing 0.05% Tween-20 and 5% fat-free dry milk for 2 h at 20 ± 5℃. The membranes were then incubated overnight with specific primary antibodies at 4℃. Subsequently, they were incubated with HRP-conjugated secondary antibodies for 1 h at room temperature, and the protein bands were visualized by using BeyoECL Plus reagent (GE Healthcare Life Sciences, UK) while β-actin was used as the loading control.
The cytotoxicity of resveratrol was estimated by using the MTT assay. Briefly, Huh7 cells were seeded in 96-well plates at a density of 5 × 103 cells per well, cultured in RPMI 1640 medium supplemented with 10% FBS, and then treated with resveratrol at varying concentrations for 48 h. Then, the cells were treated with MTT solution at 0.2 mg/mL for 3 h and incubated for 30 min at 37℃ in an atmosphere of 5% CO2. Next, the supernatants were removed and the formazan crystals that had formed were dissolved with dimethyl sulfoxide. Finally, the absorbance of the solution at 540 nm was determined by using a microplate reader (Tristar LB 941; Berthold Technologies, Germany).
The Huh7 cells were seeded into 6-cm dishes at a density of 1.5 × 105 cells per dish and cultured in RPMI 1640 medium containing 10% FBS. The cells were treated with resveratrol at varying concentrations before trypsinization and centrifuged at 500 × g at 4℃ for 2 min. The supernatants were removed, the pellets were washed twice with phosphate-buffered saline, and the cells were fixed with cold 70% ethanol (v/v) overnight at −20℃. Next, the cells were stained with PI solution containing RNase (0.2 mg/mL) and analyzed by using a Guava easyCyte Flow Cytometer (Millipore, USA). In each group, 10,000 cells were analyzed.
The Huh7 cells were seeded into 6-cm dishes at a density of 1.5 × 105 cells per dish, cultured in RPMI 1640 medium containing 10% FBS, and treated with resveratrol at varying concentrations. Total RNA was isolated by using RNA-Bee reagent (Tel-Test, USA) following the manufacturer's instructions, and cDNA was synthesized by using a reverse transcription system purchased from Promega (USA). The cDNA and specific primers were mixed with the Maxima SYBR Green qPCR master mix (Fermentas, Lithuania). The primer sequences were as follows: cyclin D1 (NM 007631.2), forward, 5′-agcagaagtgcgaagaggagg-3′ and reverse, 5′-ggcagtcaagggaa tggtctc-3′ and β-actin (X03672) forward, 5′-tgtccaccttccagca gatgt-3′ and reverse, 5′-agctcagtaacagtccgcctaga-3′. The primers were obtained from Bioneer (Korea).
The animal protocol used in this study was reviewed and approved by the ethics committee of the Seoul National University (SNU-IACUC). A mouse xenograft model using 6-week-old female BALB/c (nu/nu) mice (Orient, Korea) was established.
Tumor cells (5 × 106 Huh7-HBx cells) were suspended in 0.2 mL of serum-free DMEM and injected subcutaneously into the right flank of each nude mouse. When the tumor size reached approximately 100 mm3 (after 21 days), the mice were randomly divided into a vehicle-treated group, and 50 mg/kg and 100 mg/kg resveratrol-treated groups (n = 3 per group). For the two resveratrol-treated groups, the nude mice were injected intraperitoneally with resveratrol starting at 21 days after tumor cell inoculation. Intraperitoneal injections were given daily for 3 weeks. The vehicle-treated group was injected with normal saline. Body weight and tumor size were measured every 3 days during the 21 days of treatment. Tumor volume (V) was calculated using the following formula:
where a and b represent the long diameter (mm) and perpendicular short diameter (mm) of the tumor, respectively.
After 21 days of treatment, the experiments were ended and the mice were sacrificed by using CO2. The tumors were sampled and weighed, and the tumor growth inhibition rate was calculated by using the following formula:
where W represents tumor weight.
Tumors were removed from mice and sliced into pieces of 0.05 g each and homogenized with RIPA lysis buffer [10 mM Tris-Cl (pH 7.1), 100 mM NaCl, 1 mM EGTA, 10% glycerol, 0.5% Triton X-100, protease inhibitor cocktail (Roche, Switzerland), and phosphatase inhibitor cocktail (Sigma-Aldrich, USA)]. Samples were centrifuged at 16,000 × g and supernatants were collected and separated by SDS-PAGE. The separated proteins were transferred to nitrocellulose membranes (Pall). Western blot analysis was conducted as described above.
Tumors were removed from mice, fixed in 10% buffered formalin, and embedded in paraffin. For pathologic examination, 4-µm-thick tissue sections were stained with hematoxylin and eosin (H&E).
Formalin-fixed paraffin sections were hydrated, and heat-mediated antigen retrieval was carried out when necessary. The sections were incubated with primary antibody overnight at 4℃.
Results are expressed as the mean ± SD of at least three independent experiments. Statistical analysis was performed by using SPSS Statistics software (ver. 22; SPSS, USA). Student's t-test was used to compare differences between two groups. One-way ANOVA was used for comparing differences among multiple groups with post-hoc comparisons performed by using the Tukey's HSD test. Differences were considered significant at p < 0.05.
First, we examined HBx expression in Huh7-CMV and Huh7-HBx cells and in liver tissues isolated from HBx transgenic mice. As expected, Huh7-HBx but not Huh7-CMV cells expressed HBx. Expression in the HBx transgenic mice was verified by PCR using specific primer sets (panel A in Fig. 1). In addition, the expression of survivin, a representative hepatocarcinoma biomarker, was strongly induced in Huh7-HBx cells and in primary hepatocytes isolated from HBx transgenic mice. Therefore, HBx is coupled with the expression of survivin and the increased expressions are indicative of HCC (panel B in Fig. 1).
We evaluated the anti-proliferative effect of resveratrol by using the Huh7-HBx HCC cell line. Compared to day 0, the number of Huh7-HBx cells was increased by 2.5-fold on day 2. Resveratrol treatment at 100 µM decreased the Huh7-HBx cell number to 67% of the untreated number on day 2 (panel A in Fig. 2). To investigate whether the effect of resveratrol was specific to Huh7-HBx cells, we also conducted the MTT assay in Huh7-CMV cells. The results showed that resveratrol decreased the viability of both Huh7-CMV and Huh7-HBx cells (panels A and B in Fig. 2), indicating that the effect was not confined to HBx-overexpressing cells.
Next, we determined whether the expression of survivin protein was affected by resveratrol in Huh7-HBx cells. Consistent with the MTT assay results, western blot analysis showed that 100 µM of resveratrol decreased survivin protein expression (panel C in Fig. 2). Collectively, these results indicate that resveratrol decreases cell viability and survivin expression in Huh7-HBx HCC cells.
We considered two hypotheses for the inhibitory effect of resveratrol against Huh7-HBx cells. First, resveratrol likely inhibits cell proliferation, and second, it may induce apoptosis. To determine whether resveratrol inhibits cell proliferation and/or induces apoptosis of Huh7-HBx cells, we conducted FACS analysis. We synchronized the cell cycle at the G1 phase by using serum starvation and induced cell cycle progression by serum stimulation with or without 100 µM resveratrol. The FACS analysis showed similar cell cycle profiles for both the control and the resveratrol-treated groups at 0 h (panels A and B in Fig. 3). However, the G0/G1 phase ratio of the control group decreased over 24 h, while the population of cells in the S and G2/M phases increased. This result indicated that serum stimulation successfully induced cell cycle progression (panels A and B in Fig. 3). In contrast to the control group, the overall cell cycle profiles of the resveratrol-treated groups did not significantly change during the 24-h treatment, indicating that resveratrol induced cell cycle arrest at the G1 phase (panels A and B in Fig. 3). Furthermore, the population of cells in the sub-G1 phase, an indicator of apoptosis, was not altered in either the control or resveratrol-treated groups. Taken together, the results led us to conclude that resveratrol decreases the viability of Huh7-HBx cells by inhibiting cell proliferation, not by inducing apoptosis.
After determining that resveratrol induces G1 phase arrest, we evaluated its effects on the expression of cell cycle progression-related proteins in Huh7-HBx cells. Cyclin D1 is a well-known protein responsible for G1/S transition [27], and therefore, we evaluated its expression in resveratrol-treated Huh7-HBx cells. Western blot analysis showed that resveratrol decreased the protein expression of both cyclin D1 and survivin (panel A in Fig. 4). Next, to determine whether resveratrol inhibits cyclin D1 protein transcription, we examined its effects on mRNA expression of cyclin D1. The qRT-PCR analysis showed that resveratrol decreased mRNA expression of cyclin D1, indicating that it inhibits cyclin D1 protein transcription in Huh7-HBx cells (panel B in Fig. 4).
Western blot analysis revealed that resveratrol decreased the phosphorylation of Akt and p70S6K, which is a downstream protein of the Akt pathway (panel A in Fig. 5). However, the phosphorylation of ERK and its downstream protein p90RSK were not altered (panel B in Fig. 5), indicating that resveratrol decreased cyclin D1 protein transcription through blockade of the Akt signaling pathway. Taken together, the results led us to conclude that resveratrol inhibits the proliferation of Huh7-HBx cells by decreasing cyclin D1 protein transcription via blockade of the Akt signaling pathway.
To investigate the effect of resveratrol on xenograft model mice implanted with Huh7-HBx cells, we observed the effect of intraperitoneal injection of resveratrol on the clinical status of and tumor volume in implanted mice after growing tumors to approximately 100 mm3 for 21 days (Fig. 6).
The tumor volume in the vehicle-treated group was increased at day 21 of the experiment. Resveratrol, at doses of 50 mg/kg and 100 mg/kg, markedly reduced the tumor volumes (1,626.2 ± 152.26 mm3, p < 0.01 and 1,321.96 ± 1,024.80 mm3, p < 0.01, respectively) from that of the vehicle-treated group (4,604.96 ± 198.05 mm3) (panel A in Fig. 7). Tumor weight was also increased in the vehicle-treated group at day 21. Resveratrol at a dosage of 100 mg/kg significantly reduced tumor weight (3.61 ± 2.63 g, p < 0.05) (panel B in Fig. 7). At day 21, mice implanted with tumor cells showed prominent tumor development (panel C in Fig. 7). Compared to the vehicle-treated group, resveratrol markedly inhibited tumor development. The mean inhibition rates on day 21 were 32.2% ± 9.69% and 53.3% ± 34.74% for 50 mg/kg and 100 mg/kg resveratrol, respectively (panel D in Fig. 7). No significant differences in body weight between the resveratrol-treated groups and the vehicle-treated group were observed (panel E in Fig. 7), indicating that resveratrol is nontoxic in mice. Together, the results indicate that resveratrol has antitumor potential at doses of 50 mg/kg and 100 mg/kg in xenograft model mice implanted with Huh7-HBx cells.
We evaluated inhibitory effects of resveratrol in tumor specimens by undertaking pathological examination. Tumor specimens were collected at the end of the experiment and after treatment for 21 days. The specimens were evaluated using H&E staining and immunohistochemical staining for survivin. Resveratrol decreased tumor development and survivin expression in the tumor specimens from that in the vehicle-treated group (panel A in Fig. 8). The level of protein expression was checked by western blot analysis. The survivin/actin expression ratio was significantly reduced in the groups treated with 50 mg/kg and 100 mg/kg resveratrol (0.77 ± 0.06, p < 0.05 and 0.60 ± 0.03, p < 0.01, respectively) compared to that in the vehicle-treated group (1.0 ± 0.14) (panel B in Fig. 8). There were no significant differences in cyclin D1 expression between the resveratrol-treated and vehicle-treated groups (panel C in Fig. 8).
In the present study, we have demonstrated that resveratrol inhibits proliferation of Huh7-HBx cells by blocking the Akt signaling pathway. However, the detailed molecular mechanisms underlying the resveratrol-induced blockade of the Akt signaling pathway were not investigated. However, previous studies have reported these molecular mechanisms. Choi et al. [8] reported that resveratrol decreases the activity of phosphatidylinositide 3-kinase (PI3K), an upstream kinase of Akt, in vitro. Furthermore, it has been reported that inhibition of PI3K activity decreases Akt signaling [6], and numerous other studies have reported similar findings in various cell lines [1326]. Based on these previous reports, we propose that resveratrol might target PI3K activity to attenuate Akt signaling pathway in Huh7-HBx cells.
Most previous reports have demonstrated that either inhibition of cell proliferation or induction of apoptosis decreases the viability of cancer cells [141718]. Based on those results, we conducted FACS analysis to determine which of these actions mediated the effects of resveratrol. The results clearly showed that resveratrol induced cell cycle arrest at the G1 phase but did not alter the apoptotic cell population of Huh7-HBx cells. This result is inconsistent with those in previous studies, as resveratrol previously has been reported to induce apoptosis and decrease cell proliferation in different cancer cells [9]. This discrepancy might be due to differences in cell culture conditions and context-dependent cellular antiproliferative and apoptotic effects of resveratrol. In addition, we suggest that resveratrol might be more effective in preventing carcinogenesis than in reducing already grown cancer cells.
Cyclin D1 and other cell cycle-related proteins have been reported to be unstable. Such cell cycle-related proteins can be temporarily regulated depending on the cell cycle status. For instance, the expression of cyclin D1 can be induced during G1/S transition and subsequently downregulated, suggesting that the protein expression of cyclin D1 is tightly regulated by a balance between cyclin D1 degradation and synthesis [3]. Therefore, our determination that resveratrol decreased cyclin D1 protein expression led us to consider two possible explanations for its effects. Resveratrol decreased cyclin D1 protein expression either by inhibiting mRNA transcription or by increasing proteasome-mediated degradation. Evaluation of the mRNA level revealed that treatment with resveratrol significantly decreased cyclin D1 mRNA expression. This result indicated that resveratrol inhibits cyclin D1 protein transcription. However, it is possible that resveratrol also regulates the degradation rate of cyclin D1; further studies are required to verify this suggestion.
Previous studies in vivo have reported that resveratrol inhibited tumor growth in nude mice bearing HepG2 cells and downregulated NF-κB and VEGF cells [37], and that it prevented the development of spontaneous HCC in HBx transgenic mice [20]. However, the mechanisms of resveratrol in HBV-induced HCC in vivo are largely unreported. Resveratrol can activate the expression of p53 [5] and downregulate MCL-1 [31], anti-apoptotic protein and survivin [25], and downstream of the STAT3 pathway [25]. Recently, acetylated STAT3 received attention as a tumor-progressing factor [19]. Survivin (encoded by BIRC5 [2]), an apoptosis protein inhibitor [10], acts as a regulator of cell division, a modulator of cell death, and a stress response factor in cell proliferation and survival [210]. Survivin regulates gene expression and protein–protein interaction by inhibition of apoptosis through multiple pathways [1]. Meanwhile, a recent study clarified that HBx induces hepatocarcinogenesis together with survivin [3840] through modulation of HBx-interacting protein, a cofactor of survivin in apoptosis suppression [39]. Moreover, HBV-induced HCC shows p53 inactivation through mutations [21] and activation of the Akt signaling pathway [12]. Thus, we hypothesized that the resveratrol mechanisms might involve survivin, p53, and the Akt signaling pathway in nude mice implanted with Huh7-HBx cells. Resveratrol obviously decreased the expression of survivin and increased that of p53 in our tumor specimens. In contrast, it had no effect on the expression of cyclin D1. These results demonstrate that resveratrol exhibits anti-tumor activities in nude mice implanted with Huh7-HBx cells by downregulating survivin and upregulating p53.
In conclusion, the results in the present study suggest an anti-carcinogenesis activity of resveratrol in Huh7-HBx cells and indicate its molecular mechanisms. Resveratrol was observed to inhibit proliferation of Huh7-HBx cells by inducing cell cycle arrest at the G1 phase. It decreased cyclin D1 expression through attenuation of the Akt signaling pathway; however, it did not block the ERK signaling pathway. Thus, this study showed that resveratrol effectively decreases cell proliferation by suppressing cyclin D1 protein expression through downregulation of the Akt signaling pathway. Although this study suggests resveratrol as a potent anticarcinogenic agent in vitro and in vivo, several further studies including in vivo experiments are needed to elucidate the exact molecular mechanisms of resveratrol's inhibitory effects. The cell proliferation-related protein cyclin D1 was not affected by resveratrol treatment. Thus, survivin may be a therapeutic target, and resveratrol could have an important role in the treatment of HBV-induced HCC.
Figures and Tables
Fig. 1
Protein expression of hepatitis B virus X protein (HBx) and survivin in HBx-overexpressing Huh7 (Huh7-HBx) cells. (A) HBx expression was detected in Huh7-HBx cells but not in cytomegalovirus (CMV)-expressing Huh7 (Huh7-CMV) cells. HBx transgenic mice were verified by polymerase chain reaction using specific primer sets. (B) Survivin protein expressions were upregulated in Huh7-HBx cells compared to that in Huh7-CMV cells, and survivin protein expression was upregulated in HBx transgenic mice compared to that in wild-type mice.
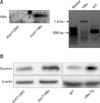
Fig. 2
Effects of resveratrol on cell proliferation and survivin expression in hepatitis B virus X protein-overexpressing Huh7 (Huh7-HBx) cells. Resveratrol inhibited cell proliferation of Huh7-HBx (A) and cytomegalovirus-expressing Huh7 (Huh7-CMV) cells (B) incubated with varying concentrations for 48 h. Results are mean ± SD of at least 3 independent experiments; **p < 0.01 comparing control group at 0 and control group at 48 h and ††p < 0.01 comparing control and resveratrol-treated groups at 48 h. (C) Resveratrol decreased survivin protein expression in Huh7-HBx cells treated with varying concentrations for 24 h. Graphs include quantification of results of at least 3 independent experiments; *p < 0.05 comparing resveratrol-treated and control groups.

Fig. 3
Effect of resveratrol on cell cycle progression in hepatitis B virus X protein-overexpressing Huh7 (Huh7-HBx) cells. Resveratrol blocked G1-S transition of Huh7-HBx cells when treated with 100 µM resveratrol for indicated times (A), as shown by FACS analysis (B). Graphs include quantification of results of at least 3 independent experiments; *p < 0.05 and **p < 0.01 comparing control and resveratrol-treated groups.

Fig. 4
Effects of resveratrol on cyclin D1 and survivin expression in hepatitis B virus X protein-overexpressing Huh7 (Huh7-HBx) cells. (A) Resveratrol decreased cyclin D1 and survivin protein expression in Huh7-HBx cells treated with varying concentrations for 8 h. Western blot analysis was conducted with β-actin as the loading control. Results represent at least 3 experiments that produced similar results. (B) Resveratrol decreased cyclin D1 mRNA expression in Huh7-HBx cells treated with varying concentrations for 4 h. Graphs include quantification of results of at least 3 independent experiments; *p < 0.05 comparing control and resveratrol-treated groups.
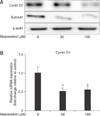
Fig. 5
Effects of resveratrol on ERK and Akt signaling pathway in hepatitis B virus X protein-overexpressing Huh7 (Huh7-HBx) cells. Resveratrol blocked Akt (A) but not the ERK signaling pathway (B) in Huh7-HBx cells treated with varying concentrations for 4 h. p-ERK, phosphorylated extracellular signal-regulated kinase.

Fig. 6
The entire schedule of the in vivo study. Tumor cells were injected subcutaneously into the right flank of each nude mouse. When the tumors' size progress approximated 100 mm3 (at 21 days), resveratrol was injected intraperitoneally daily for 3 weeks. At 21 days after treatment, the experiments were ended and the mice sacrificed. The transplanted tumors were sampled and weighed.

Fig. 7
Inhibitory effects of resveratrol in xenograft model mice implanted with hepatitis B virus X protein-overexpressing Huh7 (Huh7-HBx) cell in vivo. Huh7-HBx cells (5 × 106/0.2 mL) were inoculated into the right upper flank of BALB/c (nu/nu) mice subcutaneously. Then, the mice were randomly allocated to vehicle-treated group and two treatment groups and sacrificed at 21 days after inoculation. (A) Tumor size curve. Tumor size was measured every 3 days from day 1 to day 21. (B) Tumor weight at 21 days, the end of the study. (C) Representative photographs of nude mice bearing Huh7-HBx cells at the end of the study. (D) Inhibition of tumor growth was calculated by using the formula: inhibition rate (%) = (tumor weight of vehicle group − tumor weight of treated group)/tumor weight of vehicle group × 100%. (E) Body weight curve. Results were expressed as the mean ± SD (n = 3). Significance at *p < 0.05 and **p < 0.01 between resveratrol and vehicle-treated groups. One-way ANOVA was used for analysis with post-hoc comparisons performed by using Tukey's HSD.
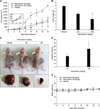
Fig. 8
Effect of resveratrol in tumor specimens. (A) Pathologic status of representative tumor specimens. Tumor specimens were taken from the xenograft model mice, embedded in paraffin and stained with hematoxylin & eosin (H&E). Expression of survivin was examined by IHC staining in the tumor tissues of vehicle- and 100 mg/kg resveratrol-treated groups. Resveratrol (100 mg/kg treated group) reduced tumor development and intensity of staining of survivin. (B) Protein expression of survivin. Xenograft model mice implanted with Huh7-HBx cells were treated with 0, 50, and 100 mg/kg resveratrol. Survivin:actin ratio was decreased in both resveratrol-treated groups. Survivin:actin expression ratio was significantly reduced in the 50 mg/kg and 100 mg/kg treated groups (0.77 ± 0.06, p < 0.05 and 0.60 ± 0.03, p < 0.01, respectively), compared to the vehicle-treated group (1.0 ± 0.14). (C) Protein expression of Cyclin D1. Cyclin D1:actin ratio did not show any significant changes. Results were expressed as the mean ± SD (n = 3). Significance at *p < 0.05 and **p < 0.01 between resveratrol- and vehicle-treated groups (Student's t-test). 400× (A).

Acknowledgments
This work was supported by the grants from the National Research Foundation of Korea (NRF-2015R1A1A1A05000 990) and Korea Mouse Phenotyping Project (KMPC; NRF-2016M3A9D5A01952417) from the Ministry of Science, ICT and Future Planning, Republic of Korea.
References
1. Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008; 8:61–70.

2. Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol. 2006; 18:609–615.

3. Bashir T, Pagano M. Aberrant ubiquitin-mediated proteolysis of cell cycle regulatory proteins and oncogenesis. Adv Cancer Res. 2003; 88:101–144.

4. Blumberg BS, Larouzé B, London WT, Werner B, Hesser JE, Millman I, Saimot G, Payet M. The relation of infection with the hepatitis B agent to primary hepatic carcinoma. Am J Pathol. 1975; 81:669–682.

5. Bobrowska-Hägerstrand M, Lillås M, Mrówczyñska L, Wróbel A, Shirataki Y, Motohashi N, Hägerstrand H. Resveratrol oligomers are potent MRP1 transport inhibitors. Anticancer Res. 2006; 26:2081–2084.
7. Chirillo P, Pagano S, Natoli G, Puri PL, Burgio VL, Balsano C, Levrero M. The hepatitis B virus X gene induces p53-mediated programmed cell death. Proc Natl Acad Sci U S A. 1997; 94:8162–8167.
8. Choi KH, Kim JE, Song NR, Son JE, Hwang MK, Byun S, Kim JH, Lee KW, Lee HJ. Phosphoinositide 3-kinase is a novel target of piceatannol for inhibiting PDGF-BB-induced proliferation and migration in human aortic smooth muscle cells. Cardiovasc Res. 2010; 85:836–844.

9. Dai Z, Lei P, Xie J, Hu Y. Antitumor effect of resveratrol on chondrosarcoma cells via phosphoinositide 3-kinase/AKT and p38 mitogen-activated protein kinase pathways. Mol Med Rep. 2015; 12:3151–3155.

10. Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 2006; 7:988–994.

11. Espinoza JL, Takami A, Trung LQ, Kato S, Nakao S. Resveratrol prevents EBV transformation and inhibits the outgrowth of EBV-immortalized human B cells. PLoS One. 2012; 7:e51306.

12. Guerrieri F, Belloni L, Pediconi N, Levrero M. Molecular mechanisms of HBV-associated hepatocarcinogenesis. Semin Liver Dis. 2013; 33:147–156.

13. Huh WB, Kim JE, Kang YG, Park G, Lim TG, Kwon JY, Song da S, Jeong EH, Lee CC, Son JE, Seo SG, Lee E, Kim JR, Lee CY, Park JS, Lee KW. Brown pine leaf extract and its active component trans-communic acid inhibit UVB-induced MMP-1 expression by targeting PI3K. PLoS One. 2015; 10:e0128365.
14. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.

15. Kew MC. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus-induced hepatocellular carcinoma. Pathol Biol (Paris). 2010; 58:273–277.

16. Knoll S, Fürst K, Thomas S, Villanueva Baselga S, Stoll A, Schaefer S, Pützer BM. Dissection of cell context-dependent interactions between HBx and p53 family members in regulation of apoptosis: a role for HBV-induced HCC. Cell Cycle. 2011; 10:3554–3565.

17. Kolodziej M, Goetz C, Di Fazio P, Montalbano R, Ocker M, Strik H, Quint K. Roscovitine has anti-proliferative and pro-apoptotic effects on glioblastoma cell lines: a pilot study. Oncol Rep. 2015; 34:1549–1556.

18. Lee DE, Lee KW, Jung SK, Lee EJ, Hwang JA, Lim TG, Kim BY, Bode AM, Lee HJ, Dong Z. 6,7,4′-trihydroxyisoflavone inhibits HCT-116 human colon cancer cell proliferation by targeting CDK1 and CDK2. Carcinogenesis. 2011; 32:629–635.

19. Lee H, Zhang P, Herrmann A, Yang C, Xin H, Wang Z, Hoon DS, Forman SJ, Jove R, Riggs AD, Yu H. Acetylated STAT3 is crucial for methylation of tumor-suppressor gene promoters and inhibition by resveratrol results in demethylation. Proc Natl Acad Sci U S A. 2012; 109:7765–7769.

20. Lin HC, Chen YF, Hsu WH, Yang CW, Kao CH, Tsai TF. Resveratrol helps recovery from fatty liver and protects against hepatocellular carcinoma induced by hepatitis B virus X protein in a mouse model. Cancer Prev Res (Phila). 2012; 5:952–962.

21. O'Dell MR, Huang JL, Whitney-Miller CL, Deshpande V, Rothberg P, Grose V, Rossi RM, Zhu AX, Land H, Bardeesy N, Hezel AF. KrasG12D and p53 mutation cause primary intrahepatic cholangiocarcinoma. Cancer Res. 2012; 72:1557–1567.
22. Parekh P, Motiwale L, Naik N, Rao KV. Downregulation of cyclin D1 is associated with decreased levels of p38 MAP kinases, Akt/PKB and Pak1 during chemopreventive effects of resveratrol in liver cancer cells. Exp Toxicol Pathol. 2011; 63:167–173.

23. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.

24. Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006; 45:529–538.

25. Quoc Trung L, Espinoza JL, Takami A, Nakao S. Resveratrol induces cell cycle arrest and apoptosis in malignant NK cells via JAK2/STAT3 pathway inhibition. PLoS One. 2013; 8:e55183.

26. Seo SG, Yang H, Shin SH, Min S, Kim YA, Yu JG, Lee DE, Chung MY, Heo YS, Kwon JY, Yue S, Kim KH, Cheng JX, Lee KW, Lee HJ. A metabolite of daidzein, 6,7,4′-trihydroxyisoflavone, suppresses adipogenesis in 3T3-L1 preadipocytes via ATP-competitive inhibition of PI3K. Mol Nutr Food Res. 2013; 57:1446–1455.

27. Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006; 24:1770–1783.

28. Su F, Schneider RJ. Hepatitis B virus HBx protein sensitizes cells to apoptotic killing by tumor necrosis factor α. Proc Natl Acad Sci U S A. 1997; 94:8744–8749.

29. Sun W, Zhong F, Zhi L, Zhou G, He F. Systematic-omics analysis of HBV-associated liver diseases. Cancer Lett. 2009; 286:89–95.
30. Tian Y, Yang W, Song J, Wu Y, Ni B. Hepatitis B virus X protein-induced aberrant epigenetic modifications contributing to human hepatocellular carcinoma pathogenesis. Mol Cell Biol. 2013; 33:2810–2816.

31. Tsutsui M, Yasuda H, Suto H, Imai H, Isobe Y, Sasaki M, Kojima Y, Oshimi K, Sugimoto K. Frequent STAT3 activation is associated with Mcl-1 expression in nasal NK-cell lymphoma. Int J Lab Hematol. 2010; 32:419–426.

32. Vastano BC, Chen Y, Zhu N, Ho CT, Zhou Z, Rosen RT. Isolation and identification of stilbenes in two varieties of Polygonum cuspidatum. J Agric Food Chem. 2000; 48:253–256.

33. Wicklow B, Wittmeier K, t' Jong GW, McGavock J, Robert M, Duhamel T, Dolinsky VW. Proposed trial: safety and efficacy of resveratrol for the treatment of non-alcoholic fatty liver disease (NAFLD) and associated insulin resistance in adolescents who are overweight or obese adolescents — rationale and protocol. Biochem Cell Biol. 2015; 93:522–530.

34. Wu CF, Yu MW, Lin CL, Liu CJ, Shih WL, Tsai KS, Chen CJ. Long-term tracking of hepatitis B viral load and the relationship with risk for hepatocellular carcinoma in men. Carcinogenesis. 2008; 29:106–112.

35. Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, Hsiao CK, Chen PJ, Chen DS, Chen CJ. Taiwan Community-Based Cancer Screening Project Group. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002; 347:168–174.

36. Yu DY, Moon HB, Son JK, Jeong S, Yu SL, Yoon H, Han YM, Lee CS, Park JS, Lee CH, Hyun BH, Murakami S, Lee KK. Incidence of hepatocellular carcinoma in transgenic mice expressing the hepatitis B virus X-protein. J Hepatol. 1999; 31:123–132.

37. Yu HB, Zhang HF, Zhang X, Li DY, Xue HZ, Pan CE, Zhao SH. Resveratrol inhibits VEGF expression of human hepatocellular carcinoma cells through a NF-kappa B-mediated mechanism. Hepatogastroenterology. 2010; 57:1241–1246.
38. Zhang W, Cai N, Ye L, Zhang X. Transformation of human liver L-O2 cells mediated by stable HBx transfection. Acta Pharmacol Sin. 2009; 30:1153–1161.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download