Abstract
It is essential to rapidly and precisely diagnose rabies. In this study, we evaluated four diagnostic methods, indirect fluorescent antibody test (FAT), virus isolation (VI), reverse transcriptase polymerase chain reaction (RT-PCR), and rapid immunodiagnostic assay (RIDA), to detect rabies in animal brain homogenates. Out of the 110 animal brain samples tested, 20 (18.2%) were positive for rabies according to the FAT. Compared to the FAT, the sensitivities of VI, RT-PCR, and RIDA were 100, 100, and 95%, respectively. The specificities of VI, RT-PCR and RIDA were found to be 100, 100, and 98.9%, respectively. Rabies viruses circulating in Korea were isolated and propagated in murine neuroblastoma (NG108-15) cells with titers ranging from 101.5 to 104.5 TCID50/mL. Although the RIDA findings did not completely coincide with results obtained from FAT, VI, and RT-PCR, RIDA appears to be a fast and reliable assay that can be used to analyze brain samples. In summary, the results from our study showed that VI, RT-PCR, and RIDA can be used as supplementary diagnostic tools for detecting rabies viruses in both laboratory and field settings.
Rabies is one of the most important viral diseases in animals and can impact human beings. It is difficult to accurately diagnose rabies in animals based on clinical symptoms such as frenzy, extreme tremors, salivation and paresis to distinguish this disease from encephalitic conditions caused by canine distemper virus or acute trauma [17]. Specific histopathologic changes in the central nervous system called Negri bodies have provided a basis for diagnosing rabies for about 100 years [17]. However, this pathological diagnostic method may be no longer suitable for providing guidance for post-exposure prophylaxis (PEP) as new diagnostic methods have been developed [10,17]. In Korea, a number of rabies cases have been recently reported in only two provinces and indirect fluorescent antibody test (FAT) for the detection of rabies has been used to analyze brain samples [4,5,11]. Recent technical advances can also provide more definitive evidence of rabies virus (RABV) infection and detect the presence of the entire virion, RABV proteins, and viral genes in infected tissue [1,2,18]. These techniques include direct visualization by electron microscopy, direct or indirect FAT, virus cultivation in cell lines, mouse inoculation test (MIT), immunohistochemistry, enzyme immunoassay, molecular hybridization, reverse transcription polymerase chain reaction (RT-PCR) including conventional, nested and real-time PCR; and demonstration of specific antibodies in cerebral fluid [1,2,6,13-15,18].
The most confirmatory test among these several diagnostic methods is virus isolation (VI) by inoculating cell cultures with brain homogenates suspected to harbor RABV. Neuroblastoma cells have been used to isolate RABV because these cells can better propagate wild RABV than other cells such as BHK21 or primary porcine kidney cells [12]. An advantage of VI is the availability of cultivated virus for further characterization by antigenic analysis. RT-PCR has been used to diagnose RABV worldwide due to its sensitivity and immense versatility, and can even be useful for examining paraffin-fixed archival and decomposed samples [3]. The nucleoprotein (N) gene of RABV is targeted for diagnosing and analyzing genetic characteristics and antigenic properties since this gene is highly conserved and associated with encapsidation of genomic RNA [20]. A rapid immunodiagnostic assay (RIDA) kit using specific monoclonal antibody against RABV can also be used for identifying RABV in brain or saliva samples from infected animals [8,9]. The RIDA is rapid and simple, and does not require any special equipment or technical expertise. In this study, we compared the ability of four diagnostic methods (FAT, VI, RT-PCR, and RIDA) to detect RABV circulating in Korea.
One hundred and ten brain samples including Ammon's horn were collected from 84 cattle, 12 raccoon dogs (Nyctereutes procyonoides koreensis), and 14 dogs between October 2008 and December 2010. The samples were obtained from Gyeonggi-do and Gangwon-do provinces (Korea) where recent rabies cases had been reported according to the National Animal Disease Database (KAHIS, Korea). Approximately 1 g of brain sample was homogenized with sand (Sigma, USA) in 10 mL of alpha-minimum essential medium (α-MEM; Gibco BRL, USA), and was centrifuged at 8,000 × g for 5 min. All homogenate samples were stored at -70℃ until used. In addition, the Ammon's horns samples were frozen until analyzed by the FAT.
The FAT was performed according to the procedure described by the Office International des Épizooties (OIE) and World Health Organization (WHO) [10,19]. In brief, frozen thin sections of Ammon's horn tissue on slides were fixed in cold acetone (-20℃) for 20 min. After three successive wash with phosphate buffer saline (PBS, pH 7.2), the slides were incubated with a monoclonal antibody (JenoBiotech, Korea) against rabies for 45 min at 37℃, and then stained with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgA, IgG and IgM (KPL, USA). After rinsing with PBS, the slides were air-dried and mounting buffered glycerin (Southern Biotechnology Associate, USA) was applied. The slides were examined under cover slips at magnification of 400 using a fluorescent microscope (Nikon, Japan). Positive and negative controls were run together with the test samples. The slide showing specific fluorescence was confirmed as positive.
Murine NG108-15 neuroblastoma cells (ATCC HB-12317) were used for virus isolation. NG108-15 cells were cultured in α-MEM supplemented with antibiotics (100 IU/mL penicillin, 10 µg/mL streptomycin, and 0.25 µg/mL amphotericin B) and 10% heat-inactivated fetal bovine serum (Gibco BRL, USA). Cell culturing was performed in 24-well plates at 37℃ in a 5% CO2 incubator. About 10% of the brain homogenate materials were centrifuged at 8,000 × g for 5 min. The supernatant was filtered through a 0.45 µm membrane (Millipore, USA) and 100 µL of filtrate was used to inoculate a monolayer of cells; the cells were then incubated at 37℃ for 1 h. After 1 h the inoculation medium was removed and replaced with fresh α-MEM. The cells were observed for cytopathic effects (CPE) for 5 days. The supernatants were then removed and the cells were fixed with 80% chilled acetone for 15 min. For the staining, the cells were incubated with a monoclonal antibody (JenoBiotech, Korea) specific for RABV for 45 min and then stained with FITC-conjugated goat anti-mouse IgA, IgG and IgM (KPL, USA). After washing in PBS, the cells were examined by fluorescent microscopy (Nikon, Japan). Titration of wild-type RABV isolates propagated in NG108-15 cells was also done by indirect fluorescent assay (IFA) mentioned above. Viral titers were determined by 50% tissue culture infectious dose per mL (TCID50/mL).
Viral RNA was extracted from brain samples using an RNA extraction kit (Qiagen, Germany) according to the manufacturer's instructions. The extracted RNA was eluted in 50 µL of RNase- and DNase-free water. RT-PCR was carried out to detect RABV genomic sequences using specific primer sets (RVNDF and RVNDR) that amplify the N gene of RABV. The primer set sequences are listed in Table 1. RT-PCR was performed in a reaction mixture containing 5 µL of denatured RNA, 1 µL of each primer (50 pmol), 5 µL of 5 × buffer (12.5 mM MgCl2), 1 µL of dNTP mix, 1 µL of an enzyme mix (reverse transcriptase and Taq polymerase), and 11 µL of distilled water (Qiagen, Germany). The cycling program was as follows: cDNA synthesis at 42℃ for 30 min followed by 45 cycles of 95℃ for 15 sec, 55℃ for 15 sec, and 72℃ for 15 sec, and a final extension at 72℃ for 5 min. The RT-PCR products were visualized by electrophoresis in 1.8% agarose gels containing ethidium bromide. Samples showing a 181 bp band were considered positive.
A commercial RIDA kit was used according to the manufacturer's instruction (Bionotes, Korea). Briefly, brain samples (about 10% of the brain homogenate materials) were individually prepared as described earlier and a swab supplied with the kit was dipped into the homogenate. The swab was transferred to the enclosed proprietary buffer designed for extraction of RABV. A 100 µL aliquot of the sample was transferred to the sample well. The final results were read 5 min after addition of the brain samples. The appearance of two lines (one was the test line and the other was the control line) was considered a positive result. The formation of one line was considered a negative result.
Sensitivity was calculated with the formula [TP/(TP+FN)] × 100 where TP was the number of samples with true-positive results as determined by the reference assay and FN was the number of samples with false-negative results. Specificity was defined as [TN/(TN+FP)] × 100 where TN was the number of samples with true-negative results and FP was the number of samples with false-positive results.
Comparison of the diagnostic methods was carried out with a total of 110 brain homogenates from 84 cattle, 14 dogs, and 12 raccoon dogs. When the 110 animal brain samples were tested, 20 were positive according to the FAT. These were designated as KRVR0801, KRVC0802, KRVR0803, KRVR0804, KRVR0901, KRVB0902, KRVB0903, KRVB0904, KRVB0905, KRVR0906, KRVB0907, KRVB0908, KRVB0909, KRVB0910, KRVB1001, KRVB1002, KRVC1003, KRVB1004, KRVC1005, and KRVB1006. The other 90 brain samples tested by the FAT were negative. Sensitivities of VI, RT-PCR, and RIDA were found to be 100, 100, and 95%, respectively. Specificities of VI, RT-PCR, and RIDA were found to be 100, 100, and 98.9%, respectively (Table 2). When testing NG108-15 cells with VI, 20 samples used to inoculate the cells induced rabies-specific fluorescence in the cytoplasm of the infected cells (Fig. 1). The samples that produced positive reactions in the FAT did not result in a distinctive CPE such as detachment in the NG108-15 cells. The wild-type RABV isolates propagated in the cells produced moderate virus titers ranging from 101.5 to 104.5 TCID50/mL (Table 3).
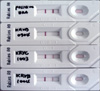
Conventional RT-PCR using a primer set that amplified the N gene of RABV was able to detect RABV in 20 samples. Various levels of the amplified gene were observed among the samples (Fig. 2). However, RT-PCR could not detect the N gene in 90 samples without any non-specific reactions. Results of the RIDA revealed that 19 samples were positive. Intensity of the test lines in the positive samples was found to vary among different field samples (Fig. 3).
Since 1993, rabies in dogs, cattle, and raccoon dogs has been observed every year in Korea. A total of 417 rabies cases were reported until 2009 [5,7]. The FAT has been recommended by the OIE and WHO, and used for diagnosing rabies worldwide [10,19]. The main advantage of the FAT is that the results of this procedure can be obtained within 3~4 h. Therefore, all rabies laboratories, including those in Korea, have routinely performed the FAT on suspected animals. Even though the FAT is most the common rapid and sensitive diagnostic test for rabies, other supplementary diagnostic methods are required when a questionable FAT result is obtained in order to arrive at a define conclusion. Any false negative results may have a catastrophic impact and false positive results can lead to unnecessary PEP. The causes of FAT errors have been traced to inadequate sampling, unsatisfactory conjugate, and lack of experience in reading the slide [2]. In the present study, we analyzed a total of 110 brain homogenates from 84 cattle, 14 dogs, and 12 raccoon dogs from the Gyeonggi-do and Gangwon-do provinces of Korea which were submitted for rabies diagnosis between 2008 and 2010. We compared the results of four different diagnostic methods (FAT, VI, RT-PCR, and RIDA) on these suspected samples.
An advantage of VI is the availability of isolated virus for further characterization by genetic analysis as well as selection of inactivated vaccine candidates. Several cell lines such as BHK21 and Vero cells have been used to propagate fixed strains, including Evelyn-Rokitnicki-Abelseth and Pasteur vaccine strains, but they are not suitable for isolating wild-type RABV [12]. It has been reported that the NG108-15 cells possessing cell membranes with acetylcholine receptors and neurotransmitter synthetic enzymes are the most susceptible to wild RABV, and cytoplasmic inclusions appear in the infected cells [2,16]. In this study, NG108-15 cells were used to isolate wild-type RABV circulating in Korea from the brain samples. Fluorescence was specifically observed in the cytoplasm of cells inoculated with the FAT-positive samples. However, all the samples that showed positive IFA reactions did not produce in a distinct CPE in the cells, suggesting that the cells infected with wild-type RABV do not develop any biological characteristics.
Many kinds of diagnostic methods in molecular biology target the nucleic acids of the causative agent. Accordingly, diagnostic methods based on RT-PCR have been used to identify viruses that cause lyssaviral diseases, including RABV, since RT-PCR has been confirmed to be a useful and sensitive method [1,14]. An important factor in determining the specificity of RT-PCR is the nucleotide sequence of the primers. The N gene, being in the conserved regions of the RABV genome, has been favored for performing RT-PCR [13]. Even if RT-PCR is capable of detecting the N gene in brain tissue of doubtful rabies cases, it is important to use fresh brain samples because viral RNA can be easily degraded through the action of ubiquitous RNases [3]. In our study, RABV was detected by RT-PCR in the 20 FAT-positive samples using primer sets that amplified the N gene. The RIDA kit is used for rapid detection of RABV in clinical samples. The sensitivity of the RIDA was also shown to be equivalent to that of the FAT [8]. FAT and RT-PCR diagnostic methods require a fluorescent microscope and thermal cycler, respectively, while VI also requires cell culture systems. However, the RIDA can be performed in less than 10 min without any special equipment or facilities. We analyzed a total of 110 samples with the RIDA kit and the results showed that 19 samples were positive. Intensity of the positive sample test lines was found to vary among different field samples, indicating that brain samples contain various viral titers.
In the present study, sensitivities of VI, RT-PCR, and RIDA relative to the FAT were shown to be 100%, 100%, and 95%, respectively. These results were similar to those of previous reports [2,6,9]. Of note, one FAT-positive isolate (KRBV0904) was also found to be positive by VI and RT-PCR but was negative according to the RIDA (98.9% specificity). The false negative RIDA result may be due to the relatively low detection limit of the kit compared to the other diagnostic methods we used. It was thus likely that this false negative sample may have had a viral titer (101.5 TCID50/mL) that was too low to be detected by the RIDA kit. Indeed, it has been reported that the detection limit of N gene-specific RT-PCR (100.5 LD50/0.03 mL) is much lower than that of the RIDA kit (101.7 LD50/0.03 mL) [6].
Based on the results from this study, VI and RT-PCR were the most sensitive diagnostic methods for detecting RABV. Despite a comparatively low sensitivity, the RIDA can also be used to rapidly analyze field samples. In conclusion, our finding demonstrated that it is possible to use these diagnostic methods to help make rapid decisions for detecting and controlling rabies.
Figures and Tables
Fig. 1
Rabies-specific fluorescence in the cytoplasm of the infected cells. Rabies field samples (KRVB1001 and KRVB1004) obtained from animals suspected of having rabies and a positive sample [Evelyn-Rokitnicki-Abelseth (ERA) strain] were used to inoculate NG108-15 cells. The cells were then stained with anti-mouse RABV nucleoprotein (N) monoclonal antibody. Uninfected NG108-15 cells stained with the anti-mouse RV N monoclonal antibody were used as a negative control and did not produce any florescence. A: KRVB1001, B: KRVB1004, C: normal NG108-15 cells, D: positive control (ERA strain). × 400.
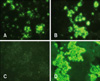
Fig. 2
Result of RT-PCR using primer sets which amplified the nucleoprotein gene of rabies virus. M: 100 bp DNA ladder, Lane 1: KRVR0801, Lane 2: KRVC0802, Lane 3: KRVR0803, Lane 4: KRVR0804, Lane 5: KRVR0901, Lane 6: KRVB0902, Lane 7: KRVB0903, Lane 8: KRVB0904, Lane 9: KRVB0905, Lane 10: KRVR0906, Lane 11: KRVB0907, Lane 12: negative control, Lane 13: positive control, Lane 14: KRVB0908, Lane 15: KRVB0909, Lane 16: KRVB0910, Lane 17: KRVB1001, Lane 18: KRVB1002, Lane 19: KRVC1003, Lane 20: KRVB1004, Lane 21: KRVC1005, Lane 22: KRVB1006, Lane 23: negative control, Lane 24: positive control.

Fig. 3
Result of the RIDA after applying the samples (ERA strain, KRVB0904, KRVC1003, and KRVB1004 isolates).
Acknowledgments
We would like to thank local veterinarians for collecting samples from animals suspected of having rabies. This work was financially supported by a grant (BAD14-2011-13-01) from the Animal and Plant and Fisheries Quarantine and Inspection Agency, Ministry for Food, Agriculture, Forestry and Fisheries, Korea.
References
1. Biswal M, Ratho R, Mishra B. Usefulness of reverse transcriptase-polymerase chain reaction for detection of rabies RNA in archival samples. Jpn J Infect Dis. 2007. 60:298–299.
2. Chhabra M, Mittal V, Jaiswal R, Malik S, Gupta M, Lal S. Development and evaluation of an in vitro isolation of street rabies virus in mouse neuroblastoma cells as compared to conventional tests used for diagnosis of rabies. Indian J Med Microbiol. 2007. 25:263–266.

3. David D, Yakobson B, Rotenberg D, Dveres N, Davidson I, Stram Y. Rabies virus detection by RT-PCR in decomposed naturally infected brains. Vet Microbiol. 2002. 87:111–118.

4. Hwang EK. Outbreak and control of rabies in animals in Korea. Korean J Vet Public Health. 1995. 19:281–293.
5. Hyun BH, Lee KK, Kim IJ, Lee KW, Park HJ, Lee OS, An SH, Lee JB. Molecular epidemiology of rabies virus isolates from South Korea. Virus Res. 2005. 114:113–125.

6. Kang B, Oh J, Lee C, Park BK, Park Y, Hong K, Lee K, Cho B, Song D. Evaluation of a rapid immunodiagnostic test kit for rabies virus. J Virol Methods. 2007. 145:30–36.

8. Lembo T, Niezgoda M, Velasco-Villa A, Cleaveland S, Ernest E, Rupprecht CE. Evaluation of a direct, rapid immunohistochemical test for rabies diagnosis. Emerg Infect Dis. 2006. 12:310–313.

9. Nishizono A, Khawplod P, Ahmed K, Goto K, Shiota S, Mifune K, Yasui T, Takayama K, Kobayashi Y, Mannen K, Tepsumethanon V, Mitmoonpitak C, Inoue S, Morimoto K. A simple and rapid immunochromatographic test kit for rabies diagnosis. Microbiol Immunol. 2008. 52:243–249.

10. Office International des Épizooties (OIE). Manual ofDiagnostic Tests and Vaccines for Terrestrial Animals. 2008. 6th ed. Paris: OIE;304–322.
11. Park YJ, Shin MK, Kwon HM. Genetic characterization of rabies virus isolates in Korea. Virus Genes. 2005. 30:341–347.

12. Rudd RJ, Trimarchi CV. Comparison of sensitivity of BHK-21 and murine neuroblastoma cells in the isolation of a street strain rabies virus. J Clin Microbiol. 1987. 25:1456–1458.

13. Rojas Anaya E, Loza-Rubio E, Banda Ruiz VM, Hernández Baumgarten E. Use of reverse transcription-polymerase chain reaction to determine the stability of rabies virus genome in brains kept at room temperature. J Vet Diagn Invest. 2006. 18:98–101.

14. Sacramento D, Bourhy H, Tordo N. PCR technique as an alternative method for diagnosis and molecular epidemiology of rabies virus. Mol Cell Probes. 1991. 5:229–240.

15. Tao XY, Niezgoda M, Du JL, Li H, Wang XG, Huang Y, Jiao Y, Cao L, Tang Q, Liang GD. The primary application of direct rapid immunohistochemical test to rabies diagnosis in China. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2008. 22:168–170.
16. Tollis M, Buonavoglia C, di Trani L, Vignolo E. Sensitivity of different cell lines for rabies virus isolation. J Vet Med B Infect Dis Vet Public Health. 1988. 35:504–508.

17. Trimarchi CV, Nadin-Davis SA. Jackson AC, Wunner WH, editors. Diagnostic evaluation. Rabies. 2007. 2nd ed. London: Academic press;411–462.

18. Wacharapluesadee S, Sutipanya J, Damrongwatanapokin S, Phumesin P, Chamnanpood P, Leowijuk C, Hemachudha T. Development of a TaqMan real-time RT-PCR assay for the detection of rabies virus. J Virol Methods. 2008. 151:317–320.

19. World Health Organization (WHO). WHO expert committee on rabies. World Health Organ Tech Rep Ser. 1992. 824:1–84.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download