Abstract
The anti-inflammatory effects of an ethanol extract of Angelica gigas (EAG) were investigated in vitro and in vivo using croton oil-induced inflammation models. Croton oil (20 µg/mL) up-regulated mRNA expression of cyclooxygenase (COX)-I and COX-II in the macrophage cell line, RAW 264.7, resulting in the release of high concentrations of prostaglandin E2 (PGE2). EAG (1~10 µg/mL) markedly suppressed croton oil-induced COX-II mRNA expression and PGE2 production. Application of croton oil (5% in acetone) to mouse ears caused severe local erythema, edema and vascular leakage, which were significantly attenuated by oral pre-treatment with EAG (50~500 mg/kg). Croton oil dramatically increased blood levels of interleukin (IL)-6 and PGE2 without affecting tumor-necrosis factor (TNF)-α and nitric oxide (NO) levels. EAG pre-treatment remarkably lowered IL-6 and PGE2, but did not alter TNF-α or NO concentrations. These results indicate that EAG attenuates inflammatory responses in part by blocking the COX-PGE2 pathway. Therefore, EAG could be a promising candidate for the treatment of inflammatory diseases.
Macrophages initiate specific immune responses against foreign antigens, and also release pro-inflammatory cytokines such as tumor-necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and others [10,13]. This process attracts an influx of lymphocytes, granulocytes, monocytes and mast cells to the site of tissue damage, which leads to tissue healing and the removal of infectious agents. TNF-α, IL-1β and IL-6 are pro-inflammatory cytokines that exert a multitude of biological activities linked to the immunopathology of acute or chronic inflammatory diseases [11,12].
TNF-α is a potent stimulator of inducible nitric oxide synthase (iNOS) gene expression in certain cell types. It also induces the chemotaxis of neutrophils and T lymphocytes and the expressions of adhesion molecules [1,46]. IL-6 is a pleiotropic inflammatory cytokine produced by T lymphocytes, monocytes, macrophages and synovial fibroblasts and plays important roles in host defense, acute phase reactions and immune responses [44]. During inflammation, activated iNOS produces high concentrations of NO [1,46,48], a regulatory molecule that has diverse physiological functions such as vasodilatation [30,31].
Another major event during the inflammatory process is the arachidonic acid cascade, which is generated from membrane phospholipids by the actions of phospholipases. Arachidonic acid is degraded by cyclooxygenase (COX) to prostaglandins (PG), which act as major physiological regulators or inflammatory mediators [35]. COX-II is a cytokine-inducible enzyme responsible for releasing high concentrations of PGE2. Both NO and PGE2 are involved in pain induction and perception [17,35].
Although steroidal and non-steroidal anti-inflammatory drugs (NSAID) are the major therapeutics used to treat inflammatory diseases, long term use of these drugs can cause adverse effects on the immune system, gastrointestinal tract, kidneys, liver, central nervous system, blood pressure and cardiovascular system [28,29,32]. Therefore, natural products with minimal adverse effects are necessary to either replace or be used in conjunction with chemical therapeutics to minimize unpredicted adverse effects.
The dried roots of Angelica gigas Nakai have been traditionally used in Oriental medicine not only for the treatment of anemia and some circulatory disorders, but also as a sedative, an analgesic, and/or a tonic agent [21]. Recently, several coumarin derivatives, including decursins, decursinol, decursinol angelate, nodakenin, nodakenetin and umbelliferone, were isolated from Angelica gigas [6,22,26]. Pharmacological effects of these derivatives include anti-tumor activities [2,18,24], anti-platelet aggregation [27], Helicobacter pylori-suppression [3] and neuroprotection [23,47].
Earlier studies showed that another species of angelica, Angelica acutiloba Kitakawa (Japanese angelica), had analgesic and anti-inflammatory effects in rodents [7,43]. Next, decursinol from Angelica gigas was found to possess analgesic and anti-nociceptive activities [9,40]. More recently, decursin was shown to inhibit the induction of inflammatory mediators from lipopolysaccharide (LPS)-stimulated macrophages [25]. Also, we previously demonstrated that an ethanol extract of Angelica gigas (EAG) suppresses the carrageenan-induced inflammatory reaction by inhibiting the TNF-α-NO pathway [41].
In this study, we assessed the efficacy of EAG on ear inflammation induced by croton oil, a phorbol ester that triggers an inflammatory reaction in a different mode from carrageenan [41]. To elucidate the mechanism of action for the EAG, we analyzed the mRNA expression of COX enzymes and their byproduct, PGE2, in a macrophage cell line.
The root of Angelica gigas was purchased from an Oriental medicine market in Seoul, Korea in 2006. The plant materials were authenticated by an expert at Chungbuk National University. The dried root (5 kg) of Angelica gigas was extracted with ethanol in an ultrasonic apparatus. Upon removal of solvent under vacuum, the ethanol EAG yielded 500 g. The extract was dissolved in 50% ethanol, and orally administered at a dose of 4 mL/kg.
The murine RAW 264.7 cell line was purchased form the American Type Culture Collection (USA), and cultured in Dulbecco's modified Eagle's medium (Sigma, USA) containing 10% fetal bovine serum and antibiotics [100 IU/mL penicillin (Sigma, USA) and 100 µg/mL streptomycin (Sigma, USA)]. The cells were incubated in a humidified 5% CO2 atmosphere at 37℃.
Cell viability after treatment with EAG was measured by the cell counting kit-8 (CCK-8; Dojindo Laboratories, Japan) [20]. RAW 264.7 cells were suspended at a final concentration of 1 × 104 cells/well and cultured in a 96-well flat-bottomed microplate. After treatment with EAG (0.01~100 µg/mL), CCK-8 (10 µL) was added to each well containing cells in 100 µL of the culture medium, and the plate was incubated for 24 h at 37℃. Viable cells were counted by absorbance measurements at 450 nm using a microplate reader. All experiments were performed in triplicate on three separate occasions.
The cells were pre-treated with EAG (1 or 10 µg/mL), and then stimulated with a combination of IFN-γ (10 IU/mL, Sigma, USA) and croton oil (20 µg/mL, Sigma, USA) for 48 h. To identify the expression of COX mRNA, reverse transcriptase-polymerase chain reactions (RT-PCR) were performed. Total RNA was isolated from RAW 264.7 cells using the Trizol method (Invitrogen, USA). The cDNA was synthesized from RNA using the Improm-II Reverse Transcription System (Promega, USA) and oliogo dT primers for a total volume of 20 µL. PCR amplification was performed using the following primers (Bioneer, Korea): COX-I sense: 5'-CCGAGAAGTACTCATGCGCCTGGT-3', COX-I antisense: 5'-CATCCTTGAAGAGCCGCAGGTGAT-3', COX-II sense: 5'-CTGACCCACTTCAAGGGAGTCTGG-3', COX-II antisense: 5'-CCATCCTTGAAAAGGCGCAGTT-3', β-actin sense: 5'-TGACCGAGCGTGGCTACAGC-3', β-actin antisense: 5'-ACCGCTCATTGCCGATAGTG-3'.
For COX-II, the PCR procedures were carried out at the pre-heating condition of 94℃ for 2 min followed by denaturation at 94℃ for 30 sec, annealing at 58℃ for 30 sec, extension at 72℃ for 30 sec for 30 cycles, and a final extension at 72℃ for 10 min. COX-I PCR procedures were carried out under identical conditions to that of COX-II, except annealing was set at 60℃ for 30 sec. The PCR products were analyzed on a 1.2% agarose gel containing ethidium bromide. The band intensities of the amplified DNAs were compared to the gel documentation system (MiniBLS Pro, Israel).
The culture supernatant was centrifuged at 30 × g for 20 min, and the presence of PGE2 was detected using a Prostaglandin E2 Enzyme Immunoassay kit (Assay Designs, USA) according to the manufacturer's instructions.
Fifty male ICR mice (5 weeks old) were purchased from the Orient-Bio (Korea). The animals were housed at the Laboratory Animal Research Center of Chungbuk National University, Korea. The animals (n = 5/cage) were maintained in a room with a constant temperature of 22 ± 1℃, relative humidity of 55 ± 10%, and 12-h light/dark cycle, and fed standard rodent chow (Samyang, Korea) and purified tap water ad libitum.
Mice (n = 10/group) were used after 1-week acclimation to the laboratory environment. The animals were orally administered EAG (50, 160 or 500 mg/kg) for 5 consecutive days. One h following the final administration, 50 µL of croton oil (5% in acetone) or its vehicle was applied to both surfaces of the ears [34].
All experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised in 1996; USA), and the protocol was approved by Institutional Animal Care and Use Committee of the Laboratory Animal Research Center, Chungbuk National University (Korea).
Five hours after croton oil application, the ears were scored for erythema according to a grading system [34], and then punctured with a cork borer (6 mm in diameter) and weighed. Separately, to quantify vascular leakage, some of the animals (n = 5/group) were intravenously injected with 5 mL/kg of Evan's blue (1% in saline) immediately before croton oil application, and the ear tissues were punched out after transcardial perfusion with cold saline (100 mL/kg). Under anesthesia with diethyl ether, the left ventricle of mice was exposed, injected with a 23G needle connected to a bottle containing saline, and perfused in a flow rate of 1 mL/min [8]. Evan's blue was extracted by immersing ear tissues in 0.5 mL of formamide at 37℃ for 3 days, and quantified at 620 nm.
Blood was collected from the abdominal vein of mice using a syringe with a 26G needle. The blood was allowed to clot at 4℃ and serum was obtained by centrifugation at 600 × g for 20 min. TNF-α and IL-6 were measured by mouse Quantikine immunoassay kits (R&D Systems, USA) using the ELISA procedures provided by the manufacturer (Molecular Devices, USA).
Serum concentration of PGE2 was measured as described above. The concentration of nitrite (NO2-), the oxidized product of NO, was measured as an indicator of NO. Serum was mixed with an equal volume of Griess reagent (1% sulfanilamide, 0.1% naphthylethylenediamide in 2.5% phosphoric acid) [16], and incubated at room temperature for 10 min. Nitrite concentration was analyzed at 540 nm, where NaNO2 was used to generate a standard curve.
As described in previous reports [9,25,40], we analyzed the ingredients in EAG with a focus on coumarin derivatives including decursin and decursinol angelate using an HPLC device (Waters, USA). Chromatographic separation was achieved using ChemcoPak Chemcobond 5-ODS-H column (3 µm, 4.6 × 150 mm internal diameter), and 2996 PDA photodiode detection was employed at a wavelength of 210 nm. The mobile phase consisted of solvent A (acetonitrile), solvent B (water), and solvent C (methanol) with the elution profile as follows: 0~10 min, 20~50% A (v/v, linear gradient), 70~45% B (v/v, linear gradient), and 10~5% C (v/v, linear gradient); 10~40 min, 50~45% A (v/v, linear gradient), 45~50% B (v/v, linear gradient), and 5% C (v/v, isocratic elution); 40~50 min, 45~100% A (v/v, linear gradient), 50~0% B (v/v, linear gradient), and 5~0% C (v/v, linear gradient). The flow rate was 1 mL/min. For quantitative determination, the EAG extract was well separated under the optimized HPLC conditions mentioned above, and the concentrations of decursin and decursinol angelate were obtained from three independent analysis.
In a cell-level analysis, EAG was not cytotoxic when exposed up to 24 h at concentrations ranging from 0.01 to 10 µg/mL, but a significant cytotoxicity (> 70%) was observed at higher concentration (100 µg/mL, p < 0.05). Because the toxicity of 100 µg/mL EAG was so large (IC50 = 46.38 µg/mL), higher concentrations were not included in this study.
Croton oil (20 µg/mL) up-regulated COX mRNA in RAW 264.7 cells (Fig. 1). Interestingly, EAG markedly suppressed croton oil-induced up-regulation of COX-II mRNA in a concentration-dependent manner, resulting in full inhibition at a concentration of 10 µg/mL. Treatment with croton oil greatly increased PGE2 production from RAW 264.7 cells, which was almost completely reversed by EAG treatment at non-cytotoxic concentrations (Fig. 2).
Application of croton oil (5% in acetone) induced severe erythema, reaching a mean score of 4.5 (maximum score 5.0), which was remarkably attenuated by the oral administration of EAG (50~500 mg/kg) in a dose-dependent manner (Fig. 3A). Croton oil increased the ear weight and vascular leakage of Evan's blue dye 1.9 and 22 fold of control levels, respectively (Figs. 3B and C). Such severe edema and vascular leakage were also significantly recovered by EAG pre-treatment.
In addition to local inflammation, croton oil application to ears also caused changes in blood cytokine and PG levels. Although croton oil did not influence the blood levels of TNF-α and NO, it increased IL-6 and PGE2 concentrations 10 and 3 times over the control values, respectively (Table 1). However, EAG significantly lowered IL-6 and PGE2 concentrations, which increased following croton oil application, without affecting the TNF-α and NO levels.
HPLC analysis of EAG revealed that decursin and decursinol angelate were the major ingredients of EAG, detected at 25.6 and 26.5 min of retention time, respectively (Fig. 4A). The concentrations of decursin and decursinol angelate in the EAG extract were 53.10 ± 0.85 and 17.94 ± 0.19%, respectively (Fig. 4B). However, the contents of other coumarin derivatives previously reported were very low.
Using an in vivo ear inflammation model, croton oil was shown to induce severe erythema and edema, indicative of vascular leakage of serum contents as confirmed by Evan's blue quantification. Among the diverse inflammatory mediators that can induce vascular permeability, it is well known that NO and PGE2 are major factors involved in the pathogenesis of many inflammation-associated diseases [14,38]. They are also known as mediators of pain induction and perception [17,35]. Therefore, TNF-α-NO and COX-II-PGE2 pathways have been considered as two main streams of inflammatory processes, which are blocked by inhibitors of iNOS (corticosteroids) and COX (NSAID), respectively [39,45]. In the present study, EAG significantly reduced erythema, edema and Evan's blue leakage at doses of 50~500 mg/kg, suggesting that it possesses suppressive activity against vascular permeability. In comparison with the maximum 30% inhibition of the erythema score, edema (ear weight) improved up to 50% with EAG pre-treatment. The improvement of edema did not correlate with the dose-response effect on vascular (Evan's blue) leakage, implying that a part of edema comes from tissue fluids other than serum, which might be also blocked by a low dose (50 mg/kg) of EAG.
In our previous study [41], we showed that carrageenan increased TNF-α and NO levels in air pouch exudate without affecting PGE2 concentration, which was later confirmed to be due to the suppression of COX mRNA expression by carrageenan (unpublished data). By comparison, croton oil greatly increased mRNA expression of COX, and consequently, PGE2 production from RAW 264.7 cells. Such results indicate that carrageenan and croton oil possess different action mechanisms for inducing inflammatory reactions.
Notably, EAG exerted anti-inflammatory effects by blocking the TNF-α-NO pathway, without influencing the PGE2 level in a carrageenan-induced inflammation model [41]. In addition to the effects observed in the carrageenan model, the suppressive activity of EAG on iNOS mRNA expression was also confirmed in LPS-activated RAW 264.7 cells (unpublished results). These results were consistent with the effects obtained with corticosteroid treatment (i.e. dexamethasone), but not by NSAID treatment (i.e. indomethacin) [34,45]. However, from our follow-up study, it seemed that the effect of EAG on the COX-II-PGE2 pathway was masked by the blocking of COX mRNA expression by carrageenan. In the case of croton oil, the PGE2 level, which increased through up-regulation of COX, was markedly attenuated by EAG, suggesting that EAG is also a COX-II-PGE2 pathway blocker. Interestingly, in this model, the TNF-α-NO pathway was masked by croton oil (data not shown). Such effects of EAG on macrophages might be due to the inactivation of signaling within the cells. The observations that decursin blocks LPS-stimulated nuclear factor-κB activation and inhibits cholesteryl ester synthesis support this hypothesis [9,33]. Therefore, it is believed that EAG affects both major inflammatory pathways involving iNOS and COX-II as suggested in the carrageenan-induced paw edema model using Hwaotang, an Angelica gigas-containing medicinal formulation [37], although it also contained extracts of at least 7 other plant species.
Unlike TNF-α and NO, blood IL-6 and PGE2 also dramatically increased following topical exposure to croton oil; however, increases in IL-6 and PGE2 were markedly suppressed by EAG. IL-6 plays important roles in acute phase reactions [15,44] and immune responses, especially in autoimmune diseases, since it enhances B lymphocyte differentiation and immunoglobulin production, including IgE [36,44]. Thus, it appears that EAG selectively affects inflammatory cells, particularly the production of cytokines by these cells. Notably, it was confirmed that EAG potentially lowered the blood IgE concentration of mice exposed to dinitrofluorobenzene (DNFB), an allergic dermatitis model (unpublished results). It is of interest to note that EAG attenuated blood IL-6 levels, which increased following local (ear) inflammation induced by croton oil, thermal burn [42] and DNFB (unpublished results), but tissue IL-6 levels were not drastically increased by carrageenan [41]. This discrepancy may be due to the different effects of the inflammatory agents on immune cells, although their mechanisms of action on the immune system are still unknown.
The broad spectrum of pharmacological effects by Angelica gigas may result from its anti-inflammatory activities. Extracts of Angelica gigas and decursinol exhibited analgesic and anti-nociceptive activities in cytokine and acetic acid-induced pain models [14,40]. The pain induced by cytokines or acetic acid results from inflammatory responses related to NO and PG. In addition, the neuroprotective and memory-enhancing effects of Angelica gigas are also due to anti-inflammatory actions, since the neurotoxins, glutamate, kainic acid and β-amyloid peptide induce oxidative stress, which thereby cause memory impairment [23,26,47]. It was confirmed that EAG contains decursin and decursinol angelate, which might be the main active ingredients for the anti-inflammatory activities in croton oil- and carrageenan-induced inflammations [41].
Although defining the exact mechanism of action requires further study, EAG markedly attenuated inflammatory signs by blocking the COX-II-PGE2 pathway in croton oil-induced inflammation. Furthermore, EAG also exerted anti-inflammatory activities against inflammation induced by carrageenan [41], thermal burn [42] and DNFB (unpublished results). These findings provide evidence that EAG could be a promising candidate or adjunct for the relief of various types of inflammation.
Figures and Tables
Fig. 1
Effects of ethanol extract of Angelica gigas (EAG) (1~10 µg/mL) on the mRNA expression of cyclooxygenase (COX)-I and COX-II in RAW 264.7 cells stimulated with interferon-γ (IFN-γ, 10 IU/mL) and croton oil (CO, 20 µg/mL). +: treated; -: non-treated.

Fig. 2
Effects of ethanol extract of Angelica gigas (EAG) (1~10 µg/mL) on prostaglandin E2 (PGE2) production from RAW 264.7 cells stimulated with IFN-γ (10 IU/mL) and croton oil (CO, 20 µg/mL). *Significantly different from IFN-γ alone (p < 0.05). †Significantly different from IFN-γ + croton oil (p < 0.05). +: treated;-:non-treat.
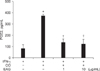
Fig. 3
Effects of 5-day pre-treatment with the ethanol extract of Angelica gigas (EAG) (50~500 mg/kg) on erythema (A), edema (ear weight, B) and vascular (Evan's blue, C) leakage of mouse ears applied with croton oil (CO, 50 µL as 5% in acetone). *Significantly different from vehicle (p < 0.05). †Significantly different from croton oil alone (p < 0.05).

Fig. 4
HPLC chromatogram (A) and content analysis (B) of decursin and decursinol angelate in ethanol extract of Angelica gigas (EAG) analyzed with a ChemcoPak Chemcobond 5-ODS-H column and eluted with acetonitrile-water-methanol gradient.

Acknowledgments
This work was supported by Priority Research Centers Program (2009-0094035) and MOEHRD (The Regional Research Universities Program/Chungbuk BIT Research-Oriented University Consortium) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology.
References
1. Adams V, Nehrhoff B, Späte U, Linke A, Schulze PC, Baur A, Gielen S, Hambrecht R, Schuler G. Induction of iNOS expression in skeletal muscle by IL-1β and NFκB activation: an in vitro and in vivo study. Cardiovasc Res. 2002. 54:95–104.

2. Ahn KS, Sim WS, Lee IK, Seu YB, Kim IH. Decursinol angelate: a cytotoxic and protein kinase C activating agent from the root of Angelica gigas. Planta Med. 1997. 63:360–361.

3. Bae EA, Han MJ, Kim NJ, Kim DH. Anti-Helicobacter pylori activity of herbal medicines. Biol Pharm Bull. 1998. 21:990–992.

4. Bresnihan B. Rheumatoid arthritis: principles of early treatment. J Rheumatol Suppl. 2002. 66:9–12.
5. Chabaud M, Fossiez F, Taupin JL, Miossec P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol. 1998. 161:409–414.
6. Chi HJ, Kim HS. Studies on the components of Umbelliferae plants in Korea: Pharmacological study of decursin, decursinol and nodakenin. Korean J Pharmacol. 1970. 1:25–32.
7. Cho S, Takahashi M, Toita S, Cyong JC. Suppression of adjuvant arthritis on rat by oriental herbs. Shoyakugaku Zasshi. 1982. 36:78–81.
8. Choi EK, Park D, Yon JM, Hur GH, Ha YC, Che JH, Kim J, Shin S, Jang JY, Hwang SY, Seong YH, Kim DJ, Kim JC, Kim YB. Protection by sustained release of physostigmine and procyclidine of soman poisoning in rats. Eur J Pharmacol. 2004. 505:83–91.

9. Choi SS, Han KJ, Lee JK, Lee HK, Han EJ, Kim DH, Suh HW. Antinociceptive mechanisms of orally administered decursinol in the mouse. Life Sci. 2003. 73:471–485.

10. Day R. Adverse reactions to TNF-α inhibitors in rheumatoid arthritis. Lancet. 2002. 359:540–541.

11. Eigler A, Sinha B, Hartmann G, Endres S. Taming TNF: strategies to restrain this proinflammatory cytokine. Immunol Today. 1997. 18:487–492.

12. Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000. 51:245–270.

13. Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996. 14:397–400.

15. Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990. 265:621–636.

16. Hevel JM, Marletta MA. Nitric-oxide synthase assays. Methods Enzymol. 1994. 233:250–258.
17. Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987. 30:103–114.

18. Jiang C, Lee HJ, Li GX, Guo J, Malewicz B, Zhao Y, Lee EO, Lee HJ, Lee JH, Kim MS, Kim SH, Lu J. Potent antiandrogen and androgen receptor activities of an Angelica gigas-containing herbal formulation: identification of decursin as a novel and active compound with implications for prevention and treatment of prostate cancer. Cancer Res. 2006. 66:453–463.

19. Jiang J, Wu F, Lu J, Lu Z, Xu Q. Anti-inflammatory activity of the aqueous extract from Rhizoma smilacis glabrae. Pharmacol Res. 1997. 36:309–314.

20. Joo SS, Yoo YM, Ahn BW, Nam SY, Kim YB, Hwang KW, Lee DI. Prevention of inflammation-mediated neurotoxicity by Rg3 and its role in microglial activation. Biol Pharm Bull. 2008. 31:1392–1396.

21. Jung DJ, Porzel A, Huneck S. Gigasol and other coumarins isolated from Angelica gigas. Phytochemistry. 1991. 30:710–712.
22. Kang SY, Lee KY, Park MJ, Kim YC, Markelonis GJ, Oh TH, Kim YC. Decursin from Angelica gigas mitigates amnesia induced by scopolamine in mice. Neurobiol Learn Mem. 2003. 79:11–18.

23. Kang SY, Lee KY, Sung SH, Kim YC. Four new neuroprotective dihydropyranocoumarins from Angelica gigas. J Nat Prod. 2005. 68:56–59.
24. Kim HH, Bang SS, Choi JS, Han H, Kim IH. Involvement of PKC and ROS in the cytotoxic mechanism of anti-leukemic decursin and its derivatives and their structure-activity relationship in human K562 erythroleukemia and U937 myeloleukemia cells. Cancer Lett. 2005. 223:191–201.

25. Kim JH, Jeong JH, Jeon ST, Kim H, Ock J, Suk K, Kim SI, Song KS, Lee WH. Decursin inhibits induction of inflammatory mediators by blocking nuclear factor-κB activation in macrophages. Mol Pharmacol. 2006. 69:1783–1790.

26. Lee JK, Choi SS, Lee HK, Han KJ, Han EJ, Suh HW. Effects of ginsenoside Rd and decursinol on the neurotoxic responses induced by kainic acid in mice. Planta Med. 2003. 69:230–234.

27. Lee YY, Lee S, Jin JL, Yun-Choi HS. Platelet anti-aggregatory effects of coumarins from the roots of Angelica genuflexa and A. gigas. Arch Pharm Res. 2003. 26:723–726.

28. Lester RS, Knowles SR, Shear NH. The risks of systemic corticosteroid use. Dermatol Clin. 1998. 16:277–288.

29. Lichtenstein DR, Syngal S, Wolfe MM. Nonsteroidal antiinflammatory drugs and the gastrointestinal tract. The double-edged sword. Arthritis Rheum. 1995. 38:5–18.

30. Marletta MA, Yoon PS, Iyengar R, Leaf CD, Wishnok JS. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1998. 27:8706–8711.

31. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991. 43:109–142.
32. Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001. 286:954–959.

33. Ohshiro T, Namatame I, Lee EW, Kawagishi H, Tomoda H. Molecular target of decursins in the inhibition of lipid droplet accumulation in macrophages. Biol Pharm Bull. 2006. 29:981–984.

34. Oliveira de Melo J, da Conceição Torrado Truiti M, Muscará MN, Bolonheis SM, Dantas JA, Caparroz-Assef SM, Cuman RK, Bersani-Amado CA. Anti-inflammatory activity of crude extract and fractions of Nectandra falcifolia leaves. Biol Pharm Bull. 2006. 29:2241–2245.

35. Pang L, Hoult JR. Cytotoxicity to macrophages of tetrandrine, an antisilicosis alkaloid, accompanied by an overproduction of prostaglandins. Biochem Pharmacol. 1997. 53:773–782.

36. Park HH, Lee S, Son HY, Park SB, Kim MS, Choi EJ, Singh TS, Ha JH, Lee MG, Kim JE, Hyun MC, Kwon TK, Kim YH, Kim SH. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch Pharm Res. 2008. 31:1303–1311.

37. Park WH, Park SY, Kim HM, Kim CH. Effect of a Korean traditional formulation, Hwaotang, on superoxide generation in human neutrophils, platelet aggregation in human blood, and nitric oxide, prostaglandin E2 production and paw oedema induced by carrageenan in mice. Immunopharmacol Immunotoxicol. 2004. 26:53–73.

38. Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM. Mechanisms of TNF-α- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest. 2003. 111:821–831.

39. Romano M, Faggioni R, Sironi M, Sacco S, Echtenacher B, Di Santo E, Salmona M, Ghezzi P. Carrageenan-induced acute inflammation in the mouse air pouch synovial model. Role of tumour necrosis factor. Mediators Inflamm. 1997. 6:32–38.

40. Seo YJ, Kwon MS, Park SH, Sim YB, Choi SM, Huh GH, Lee JK, Suh HW. The analgesic effect of decursinol. Arch Pharm Res. 2009. 32:937–943.

41. Shin S, Jeon JH, Park D, Jang JY, Joo SS, Hwang BY, Choe SY, Kim YB. Anti-inflammatory effects of an ethanol extract of Angelica gigas in a Carrageenan-air pouch inflammation model. Exp Anim. 2009. 58:431–436.

42. Shin S, Park D, Jeon JH, Kim JS, Park SK, Kim YB. Anti-inflammatory effects of an ethanolic extract of Angelica gigas in a thermal burn model. J Biomed Res. 2008. 9:29–36.
43. Tanaka S, Kano Y, Tabata M, Konoshima M. Effects of "Toki" (Angelica acutiloba Kitagawa) extracts on writhing and capillary permeability in mice (analgesic and antiinflammatory effects). Yakugaku Zasshi. 1971. 91:1098–1104.

45. Wallace JL, Chapman K, McKnight W. Limited anti-inflammatory efficacy of cyclo-oxygenase-2 inhibition in carrageenan-airpouch inflammation. Br J Pharmacol. 1999. 126:1200–1204.

46. Xie QW, Whisnant R, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon γ and bacterial lipopolysaccharide. J Exp Med. 1993. 177:1779–1784.

47. Yan JJ, Kim DH, Moon YS, Jung JS, Ahn EM, Baek NI, Song DK. Protection against β-amyloid peptide-induced memory impairment with long-term administration of extract of Angelica gigas or decursinol in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2004. 28:25–30.

48. Yui Y, Hattori R, Kosuga K, Eizawa H, Hiki K, Kawai C. Purification of nitric oxide synthase from rat macrophage. J Biol Chem. 1991. 266:12544–12547.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download