Kennel cough is one of the most significant clinical problems in dogs and ubiquitous in intensively housed animal facilities such as breeding kennels and animal shelters [8]. The causes of kennel cough have been involved in the combination of microbial agents, including viruses and bacteria, and environmental factors such as crowded conditions and other stressors [8].
Among bacteria, Streptococcus (S.) equi subsp. zooepidemicus has been recently reported in animal shelter in USA [10,11] and a research kennel in Korea [9]. These pathogen have been isolated from horses, cows, pigs, sheep, guinea pigs and domestic fowls as well as dogs, and can be transmitted between species [8]. S. equi subsp. zooepidemicus is closely related with S. equi subsp. equi, which is a causative agent of strangles in horses and dogs [4,8]. Although the identification and differentiation of the organism relies on the biochemical characteristics, the detection of specific genes has been used for a diagnostic purpose [2,3]. This report described an outbreak of acute hemorrhagic pneumonia of dogs in a shelter in Korea where approximately a thousand stray or abandoned dogs per month were taken in or out. Dogs were divided by their weight before admission in the facility.
Kennel cough in shelters has been recognized to be one of the common disorders as seen in other crowded kennels [8]. The mean mortality in this shelter has generally been managed below one percent. On December 7th of 2007, veterinarians at the shelter reported the occurrence of an unknown disorder which was mostly symptomatic as severe respiratory distress and that more than 30 dogs a day had been dead in 2 out of 5 buildings during 2 weeks. Eighty to ninety percent of dogs suffered from severe respiratory distress such as depression, cough, and lethargy. Irrespective of intensive care, 50% of the affected dogs died with evidence of nasal bleeding or hematemesis within a couple of days after clinical onset.
Necropsy was performed on two dogs which died with nasal bleeding and one euthanized dog. For histopathology, main internal organs (trachea, spleen, stomach, small and large intestine) including brain, tonsil, lung, liver, kidney and lymph nodes were collected and fixed with 10% phosphate buffered formalin solution. Tissues were routinely processed, embedded in paraffin and stained with H&E and Gram stain. Also, some of lung, liver and spleen tissues were aseptically plated on sheep blood and MacConkey agar and incubated for 48 h at 37℃ in aerobic and anaerobic conditions. Isolated bacteria were identified using API 20 strep kit (bioMérioux, France) and PCR for the sodA, seeH, seeI genes as described previously [1,2]. Antiphagocytic factor, Se18.9 was also amplified as described method by Tiwari et al. [12].
Briefly, primers FUS (5'-ATACAGGCTGAAATTGCAGG-3') and FDS (5'-CTTGCGAAAACCAGTTTAGG-3') designed from se18.9 were used to amplify chromosomal DNA in bacteria. The PCR reaction started at 92℃ for 2 min following 30 cycles of 92℃ at 1 min, 57℃ at 0.5 min and 72℃ for 4 min. A final 10 min extension step at 72℃ was carried out. Amplicon was visualized on a 2% agarose gel. Antimicrobial susceptibility test was performed by the disc diffusion method using 20 antimicrobial drugs. For viral agents, PCR was carried out to amplify the specific sequences of the canine distemper (CD) and canine adenovirus type 1 and 2 (CAV-1, CAV-2) using methods described previously by Elia et al. [5] and Hue et al. [6], respectively. Canine parainfluenza virus (CPIV) was examined by a commercial kit (Veteck CPIV; Intron, Korea). Immunohistochemistry was performed by a streptoavidin-biotin peroxidase complex (ABC) method using monoclonal and polyclonal antibodies for CD (Serotec, UK), CAV-2 (USBiological, USA) and CPIV (USBiological, USA).
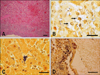
Grossly, large amounts (50~150 mL) of dark red fluids filled the thorax of all carcasses. The lungs failed to collapse and were hemorrhagic, rubbery, and appeared mottled dark red on the surface. A large amount of red frothy materials filled the trachea and large bronchi. No significant gross lesions were found in other organs. Histopathologically, hemorrhagic bronchopneumonia was accompanied with diffuse infiltration of edema fluids, inflammatory cells and bacterial colonies (Fig. 1A). Mild suppurative tracheitis was also observed. Lymphoid depletion was inconsistently shown in spleen, tonsil and bronchial lymph nodes. Gram-positive cocci were detected in blood vessel and/or parenchyma of lung (Fig. 1B), liver (Fig. 1C), spleen, kidney and cerebrum (Fig. 1D).
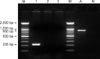
The β-hemolytic colonies were uniformly cultured on blood agar in the necropsed dogs. The isolates were identified as S. equi subsp. zooepidemicus by PCR and an API 20 strep kit. Approximately, a 0.9 Kb amplicon was amplified by the primer FUS and FDS for se18.9, antiphagocytic factor H binding protein (Fig. 2). The bacterium was susceptible to amoxicillin, ampicillin, cephalexin, doxycycline, penicillin and enrofloxacin but resistant to gentamicin, kanamycin and lincomycin. CAV-1 and 2, CD and CPIV were also screened by PCR and immunohistochemistry. None of the tested viruses were detected in any cases.
On the basis of the bacterial isolation and pathological findings, we diagnosed that the hemorrhagic bronchopneumonia was caused by S. equi subsp. zooepidemicus. The lesions were similar to those as described by previous reports [7-9,11]. However, the degree of lesions was varied across individuals. For example, even if the hemorrhage and inflammation in the lungs were generally observed, the extent of lesions was variable according to how much time had elapsed in the course of the disease. Bacterial emboli were distributed in blood vessels in the cerebrum (1/3), liver (2/3), spleen (2/3) and kidney (1/3). Lympholysis and lymphoid depletion were detected in the spleen (1/3), tonsil (2/3) and bronchial lymph node (1/3). Mild tracheitis was observed in one dog. The cause of kennel cough has been inferred to the infectious microbes and environmental stresses such as transportation and crowding [4,8]. Especially, transportation and viral infections may cause good conditions for bacterial colonization in the lung [9].
It was difficult to determine the sources of the infection due to the dogs continuously entering and leaving on a daily basis. However, it was interesting that cats had no signs even if they were reared in a neighboring building of same shelter. It was supposed that the difference of virulence factors could make it possible to cause a severe illness in dogs rather than cats. For instance, antiphagocytic factor, Se18.9 has been identified with the range from 0.8 to 4 Kb in S. equi supsp. zooepidemicus strains [12,13]. In this study, 0.9 Kb amplicon was amplified from the bacterium. It was a different size compared to the genes detected from the isolates found in a US shelter [11].
In spite of antibiotic treatments, the survival rate did not improve until follow-up measures, including the improved sanitation and depopulation program, had been implemented in this shelter. Additionally, the shelter should be managed by well-trained workers who are willing to carry out all sanitation and management procedures. Importantly, the principal respiratory signs in this case could be rapidly improved after the recruitment of new managers responsible for the operation of dog houses even if we could not prove the causative bacteria from the equipment and other materials used in the facility. Consequently, the mortality rate rapidly dropped after the improvement of personal and sanitary management. Also, it will be necessary to have the staffs get rid of all materials used in their facilities and disinfect the cages and floors equipped in buildings in order to avoid the relapse. On the other hand, the animal shelter should be consider decreasing the population in severely affected facilities and the decrease of total number of dogs in each room of the facility. In previous report [9], authors suspected a similar outbreak occurred in the private kennels that had supplied the dogs. It was confirmed that canine hemorrhagic pneumonia caused by S. equi subsp. zooepidemicus occurred in a crowded shelter in Korea.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download