Abstract
Fucoidan is a sulfated polysaccharide purified from brown algae including Fucus vesiculosus and has a variety of biological effects including mobilization of hematopoietic progenitor cells. Recently, we demonstrated that fucoidan stimulates the antigen-presenting functions of dendritic cells. In this study, we investigated the radioprotective effects of fucoidan on bone marrow cells (BMCs), which are the main cellular reservoir for the hematopoietic and immune system. To evaluate the effects of fucoidan, we assayed cell viability and immune responses. In a viability assay, fucoidan significantly increased the viability of BMCs. Based on the results of flow cytometric analysis, the increased viability of fucoidan-treated BMCs was attributed to the inhibition of radiation-induced apoptosis. Furthermore, fucoidan altered the production of immune-related cytokines from BMCs and increased the capability of BMCs to induce proliferation of allogeneic splenocytes. Taken together, our study demonstrated that fucoidan has radioprotective effects on BMCs with respect to cell viability and immunoreactivity. These results may provide valuable information, useful in the field of radiotherapy.
Bone marrow contains a variety of cells that are required to maintain the hematopoietic and immune systems. On exposure to ionizing radiation, bone marrow is profoundly damaged and severe immunosuppression occurs. Therefore, protection of bone marrow against gamma radiation is extremely important in individuals in danger of radiation exposure.
To protect the host against the harmful effects of radiation exposure, radioprotective agents have been developed over several decades [5]. Although the radioprotective agent amifostine has been used in clinical settings [17,22], it generates serious side effects, including nausea, probably due to its synthetic nature [15]. Recent studies have focused on the development of radioprotective agents derived from natural sources and that display minimal side effects on normal cells [1]. For example, some polysaccharides purified from herbs have been shown to have radioprotective and immunostimulating effects on host immune cells [9,21].
Fucoidan is a sulfated polysaccharide purified from brown algae, such as Fucus vesiculosus, and has been shown to have a variety of biological effects [2,3]. Previous studies have indicated that fucoidan stimulates the mobilization of hematopoietic progenitor cells (HPCs) from their niche within bone marrow to peripheral blood via inhibition of the selectin-mediated adhesion of HPCs [3]. In addition, fucoidan-induced mobilization of HPCs is associated with increased plasma levels of stromal-derived factor-1 in vivo [19]. Recently, we demonstrated that fucoidan stimulates multiple functions of dendritic cells (DCs), which are potent antigen-presenting cells (APCs) in the immune system [10].
Although the biological functions of fucoidan in hematopoietic and immune systems have been studied for many years, its radioprotective effects on bone marrow cells (BMCs) have not been elucidated. In this study, we investigated the protective effects of fucoidan on irradiated BMCs. Specifically, we quantified the viability of cells, cytokine production, and allostimulatory capability of this agent.
C57BL/6 and Balb/c mice were purchased from Orient Bio (Korea) and maintained at our animal facility. Seven to 12 week-old female mice were used in this study. All experiments using animal were performed based on the institutional guideline of Cheju National University for laboratory animal use and care (Approval No: 20070005). Fucoidan, originated from Fucus vesiculosus, was obtained from Sigma (USA) and dissolved in phosphate-buffered saline (Invitrogen, USA). The endotoxin level of the fucoidan preparation was less than 0.1 EU/ml from QCL-1000 Chromogenic LAL endpoint assay (Lonza Walkersville, USA) according to the manufacturer's protocol.
BMCs were harvested from the femurs and tibias of mice of C57BL/6 mice as described in previous report [9]. Any contaminated red blood cells were eliminated by ammonium chloride-potassium carbonate lysis buffer. To culture BMCs, RPMI 1640 media containing 5% fetal bovine serum (FBS), 2 mM L-glutamine, and 100 U/ml penicillin/ streptomycin (Invitrogen, USA) was used. For the cell viability assay, BMCs were cultured at a concentration of 2 × 105 cells/well in 96-well plates and treated with fucoidan before irradiation. The cultured wells were incubated with 10 µl/well of Cell Counting Kit-8 solution (CCK-8 solution; Dojindo, USA) for 4 h and the optical density (O.D.) of wells was measured at 450 nm by using a microplate reader (Molecular Devices, USA).
The BMCs were irradiated using a 60Coγ-ray source (MDS Nordion C-188 standard source) established in the Applied Radiological Science Research Institute, Cheju National University (Korea). Irradiation on cells was performed once at 1 Gy for this study.
Fucoidan (50 µg/ml) was administrated in 1 × 106 cells/ml of BMCs for 24 h and then single-exposed to gamma irradiation. After 24 h, the supernatants were harvested and used for the determination of IL-12 and TNF-alpha, the representative cytokines of cell-mediated and innate immunity. The cytokine concentration of supernatant were measured by enzyme-linked immunosorbent assay (ELISA) using CytoSet antibody pairs (Biosource International, USA) based on the manufacturer's manual.
BMCs were cultured at a concentration of 1 × 106 cells/ml in 6-well plates and treated with fucoidan (50 µg/ml) for 24 h and then irradiated once (1 Gy). After 24 h, the cells were harvested and stained for flow cytometric analysis as described in our previous report [9]. Briefly, we used biotin-labeled anti-Gr-1, anti-I-Ab (MHC II), anti-CD86 (B7.2) monoclonal antibody as the primary antibody and streptavidin-fluorescein isothiocyanate (FITC) as the secondary antibody (BD Biosciences, USA). For the measurement the percentage of apoptotic cells, cells were stained by using annexin V-FITC/propidium iodide (PI) kit (Biosource International, USA) as described in the manufacturer's instructions. For the measurement of mitochondrial membrane potential, cells were incubated with 10 µg/ml rhodamine 123 (Sigma, USA) for 30 min. All stained cells were analyzed by using FACSCalibur and CellQuest (Beckton Dickinson, USA).
BMCs were cultured and treated as we described in flow cytometric analysis. Splenocytes obtained from Balb/c mice were used as allogeneic responder cells for the co-culture with BMCs of C57BL/6 mice as described in previous report [9]. 2 × 105 cells/well allogeneic splenocytes were co-cultured with 1,852~5 × 104 cells/well BMCs in 96-well culture plates for 5 days. To minimize the proliferation of BMCs themselves in total value, all BMCs were irradiated immediately prior to co-culture. The culture medium was RPMI 1640 medium containing 10% FBS, 0.1 mM non-essential amino acid, 1 mM sodium pyruvate, 2 mM L-glutamine, 100 IU/ml penicillin/streptomycin, and 50 µM 2-mercaptoethanol (Invitrogen, USA). The co-cultures were pulsed with 1 µCi/well 3H-thymidine (PerkinElmer, USA) for last 18 hrs and the incorporated radioactivity in cells was measured by a liquid scintillation counter (Wallac Microbeta TriLux; PerkinElmer, USA).
BMCs were cultured and treated as previously described in flow cytometric analysis section. The cells were harvested and then used for Western blot analysis. The lysates of treated BMCs were obtained and the Western blot analysis was performed as described in a previous report [8]. Briefly, proteins blotted on nitrocellulose membrane were probed with anti-Bcl-2, Bcl-xL, Bax antibody (Santa Cruz Biotechnology, USA), anti-cIAP-1, cIAP-2 antibody (Millipore, USA), and anti-β-actin antibody (Sigma, USA) and sequentially appropriate horseradish peroxidase-labeled secondary antibodies. The blot was developed by SuperSignal West Pico Cheminluminescent Substrate (Pierce Biotechnology, USA).
To examine the effects of fucoidan on BMC viability, we cultured BMCs at a concentration of 2 × 105 cells/well in 96-well plates and treated them with fucoidan before irradiation (Fig. 1). After culturing the cells, CCK-8 solution was added and the O.D. of wells was measured. In the absence of fucoidan, control BMCs showed significantly higher viability than irradiated BMCs (p<0.05). Fucoidan significantly increased the viability of control BMCs within the range of concentration (2-50 µg/ml) and in irradiated BMCs (10-50 µg/ml). BMCs were cultured without cytokines, which may maintain their survival in vivo. Thus, it was likely that this manipulation would lead to cytokine withdrawal-induced cell death. Irradiation significantly decreased the BMC viability. Together, these results suggest that fucoidan may protect BMCs from cytokine withdrawal-induced cell death and irradiation.
For this test, BMCs were cultured at a concentration of 1 × 106 cells/ml in 6-well plates and treated with fucoidan (50 µg/ml) for 24 h and then irradiated once (1 Gy). After 24 h, cells were harvested and used for the cell death measurements. Annexin V-FITC/PI staining and rhodamine 123 staining were used to confirm the protective effects of fucoidan on the viability of irradiated BMCs (Fig. 2). With the annexin V-FITC/PI staining (Fig. 2A), we found that fucoidan consistently increased the number of viable cells, the double-negative cells (annexin V-/PI-). Specifically, cell viability was only 15% with irradiation, which increased to 62% with fucoidan treatment. In addition, fucoidan decreased the ratio of late apoptotic cells (annexin V+/PI+).
Mitochondria are known to play a critical role in the apoptotic process of cells. Thus, we performed rhodamine 123 staining to determine mitochondrial potential as cellular apoptosis decreases the stability of mitochondrial potential (Fig. 2B). Our results indicated that irradiation decreased mitochondrial potential; however, this was recovered by fucoidan treatment. Taken together, our results demonstrate that fucoidan increases BMC viability following irradiation. These effects may be the result of apoptotic inhibition in irradiated BMCs.
To further investigate the functional role of fucoidan, we examined the expression levels of apoptosis-related molecules in BMCs. Western blot analysis (Fig. 3) indicated that the expression levels of Bcl-2, Bax, and cIAP-1 were increased in fucoidan-treated BMCs. In densitometry results (B), Bcl-2 and Bax expression was increased in all fucoidan-treated BMCs whereas cIAP-1 expression was increased only in fucoidan-treated BMCs. However Bcl-xL was not detected in these experiments.
BMCs are one of the main immune cell reservoirs in hosts. To determine the recovery effects of fucoidan on the immune function of irradiated BMCs, we measured the expression levels of some important cell surface markers. By flow cytometric analyses (Fig. 4), we found that a phenotypic marker for granulocytes, Gr-1 expression, was significantly increased by fucoidan treatment. In contrast, the expression of immune-related markers, B7.2 (data not shown) and MHC II, were not significantly altered. Our results demonstrated that fucoidan significantly increases the expression of a granulocyte marker on BMCs, suggesting that this agent may facilitate proportional changes of cell types in BMCs and preferentially protect granulocytes in BMCs from growth factor-withdrawal or irradiation induced cell death.
We investigated whether fucoidan-treated BMCs produce high levels of cytokines related to immune responses. IL-12 and TNF-alpha were selected for this study as they are representative cytokines which are involved in cellmediated and innate immunity, respectively. In ELISA (Fig. 5), fucoidan significantly increased the production of IL-12 in fucoidan-treated BMCs compared to control BMCs and increased the production of TNF-alpha in fucoidan-treated BMCs and fucoidan-treated irradiated BMCs compared to control BMCs and irraditated BMCs respectively. However, any significant increase of IL-12 was not detected in fucoidan-treated irradiated BMCs. These results suggest that fucoidan may alter cytokine production as well as cell viability.
The capacity of BMCs to activate the proliferation of allogeneic splenocytes was examined to determine whether fucoidan alters these immune responses. BMCs include various types of APCs as precursors or matured cells. Using MLR assays, BMCs of C57BL/6 mice were used as stimulators and splenocytes of Balb/c mice were used as allogeneic responder cells. To measure the proliferation of allogeneic splenocytes alone, all BMCs were irradiated immediately prior to co-culture. Fucoidan-treated BMCs and fucoidan-treated irradiated BMCs were found to have a significantly increased capability to enhance the proliferation of allogeneic responder cells compared to control BMCs and irradiated BMCs respectively (Fig. 6). These results suggest that fucoidan treatment significantly up-regulates APC functions of BMCs.
Fucoidan is known to have a variety of biological functions, and the immunomodulatory activity of this agent has been studied extensively for many years. Recent studies demonstrated that fucoidan has significantly important biological effects on natural killer cells [13], hematopoietic stem cells [6], endothelial cells [11], and DCs [10]. Although the effects of fucoidan on the mobilization of HPCs in bone marrow have been studied well, there are few studies about the direct effects of fucoidan on BMCs. In this study, we investigated the radioprotective effects of fucoidan on BMCs by examining cell viability and immunostimulatory activity.
Research on the development of radioprotective agents has focused on natural plant-derived compounds as potential candidates; medicinal herbs, including ginseng, are the main sources for the purification of effective candidates [7,18]. In a previous study, we demonstrated that ginsan, a polysaccharide from Panax ginseng, has radioprotective effects on BMCs [9]. Some biological response modifiers, including those consisting of polysaccharides, have been shown to have radioprotective and immunostimulatory effects. Immunostimulatory signaling is thought to transduce the survival signal in immune cells and is then manifested as radioprotective activity.
On flow cytometric analysis, fucoidan-treated BMCs showed higher levels of surface Gr-1 expression than controls. As bone marrow is the main cellular source for the hematopoietic and immune systems, it contains large numbers of lymphocytes, granulocytes, and stromal cells as precursors and mature cells. Our results suggested that a specific population of BMCs, such as Gr-1+ granulocytes, may selectively survive in response to fucoidan treatment following irradiation. Future research should focus on determining which cell types are selected by fucoidan and the mechanism of action of this agent.
To investigate how fucoidan protects BMCs from cell death, the expression levels of apoptosis-related molecules were measured by Western blot analysis. Among the molecules belonging to the Bcl-2 family, Bcl-2, Bcl-xL, and Bax were selected due to their connection to apoptotic pathways involving mitochondria [4,14]. In addition, the levels of expression of cIAP-1 and cIAP-2, other anti-apoptotic molecules [12,16,20] were examined in the same experiments. We found that the levels of expression of Bcl-2, Bax, and cIAP-1 were higher in fucoidan-treated BMCs as compared with controls; there were no differences in cIAP-2 expression between treated and control BMCs. Bcl-2 and Bax proteins show anti-apoptotic and pro-apoptotic effects, respectively. However, both were upregulated in the present study. It is possible that enhanced Bcl-2 expression may compensate for the activity of Bax and other anti-apoptotic molecules, such as cIAP-1 also may support the process. And also, there is another possibility that fucoidan may induce the cell death of some specific cell types whereas it enhances the survival of other cell types of BMCs. The effects of fucoidan on specific cell types of BMCs need to be investigated in future work.
Our findings indicated that fucoidan-treated BMCs have an increased capability to stimulate allogeneic splenocytes as compared with controls. As fucoidan increased cytokine production, but not immune-related surface markers, it is postulated that the observed up-regulation of immune responses may be due primarily to increased cytokine production and a higher percentage of Gr-1+ cells, including APCs within stimulator cells.
Taken together, our results demonstrate that the sulfated polysaccharide fucoidan has radioprotective effects on BMCs. These effects include aspects of cell viability and immunomodulatory activity. In conclusion, the results of this study may facilitate the development of new radioprotective agents with reduced toxicity.
Figures and Tables
Fig. 1
Fucoidan increases bone marrow cell (BMC) viability. Asterisks (*, **, ***) indicate p < 0.05, 0.01, 0.001 vs Non-irradiation (NO-IR) control (fucoidan 0 µg/ml) and ##, ### indicate p < 0.01, 0.001 vs irradiation (IR) control (fucoidan 0 µg/ml), respectively.

Fig. 2
Inhibition of bone marrow cell (BMC) apoptosis by fucoidan treatment. (A) Numbers indicate the cell percentage of quadrant. (B) Numbers indicate the mean fluorescence intensity of all cells and brackets include the percentage of high expressed cells (M1).

Fig. 3
The altered expression of apoptosis-related molecules in fucoidan-treated bone marrow cells. (A) Western blot. (B) Densitometry of western blot.
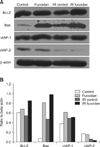
Fig. 4
Surface marker expression was up-regulated on bone marrow cells. (A) Numbers indicate the percentage of high expressed cells (M1) compared to that of fluorescence control. (B) Percentages of high expressed cells were analyzed statistically. An asterisk (*) indicate p < 0.05 vs control. A sharp (#) indicate p < 0.05 vs IR control.

Fig. 5
Fucoidan treatment enhances cytokine production of bone marrow cells (BMCs). The supernatants of BMCs were collected and ELISA was performed to quantify cytokine concentrations. N/D: non-detectable level of cytokine. Asterisks (***) indicate p < 0.001 vs control. Sharps (###) indicate p < 0.001 vs IR control.

Fig. 6
Fucoidan-treated bone marrow cells (BMCs) increases the proliferation of allogeneic splenocytes. Allogeneic splenocytes (2 × 105 cells/well) were co-cultured with BMCs in 96-well culture plates. Asterisks (**, ***) indicate p < 0.01, 0.001 vs control, respectively. Sharps (###) indicate p < 0.001 vs IR control.

Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2004-202-E00184) and performed under the program of Basic Atomic Energy Research Institute which is a part of the Nuclear R&D Programs funded by the Ministry of Science & Technology of Korea.
References
1. Arora R, Gupta D, Chawla R, Sagar R, Sharma A, Kumar R, Prasad J, Singh S, Samanta N, Sharma RK. Radioprotection by plant products: present status and future prospects. Phytother Res. 2005. 19:1–22.

2. Choi EM, Kim AJ, Kim YO, Hwang JK. Immunomodulating activity of arabinogalactan and fucoidan in vitro. J Med Food. 2005. 8:446–453.
3. Frenette PS, Weiss L. Sulfated glycans induce rapid hematopoietic progenitor cell mobilization: evidence for selectin-dependent and independent mechanisms. Blood. 2000. 96:2460–2468.

4. Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999. 13:1899–1911.

5. Hosseinimehr SJ. Trends in the development of radioprotective agents. Drug Discov Today. 2007. 12:794–805.

6. Irhimeh MR, Fitton JH, Lowenthal RM. Fucoidan ingestion increases the expression of CXCR4 on human CD34+ cells. Exp Hematol. 2007. 35:989–994.

7. Ivanova T, Han Y, Son HJ, Yun YS, Song JY. Antimutagenic effect of polysaccharide ginsan extracted from Panax ginseng. Food Chem Toxicol. 2006. 44:517–521.

8. Joo HG, Goedegebuure PS, Sadanaga N, Nagoshi M, von Bernstorff W, Eberlein TJ. Expression and function of galectin-3, a β-galactoside-binding protein in activated T lymphocytes. J Leukoc Biol. 2001. 69:555–564.
9. Kim HJ, Kim MH, Byon YY, Park JW, Jee Y, Joo HG. Radioprotective effects of an acidic polysaccharide of Panax ginseng on bone marrow cells. J Vet Sci. 2007. 8:39–44.

10. Kim MH, Joo HG. Immunostimulatory effects of fucoidan on bone marrow-derived dendritic cells. Immunol Lett. 2008. 115:138–143.

11. Lake AC, Vassy R, Di Benedetto M, Lavigne D, Le Visage C, Perret GY, Letourneur D. Low molecular weight fucoidan increases VEGF165-induced endothelial cell migration by enhancing VEGF165 binding to VEGFR-2 and NRP1. J Biol Chem. 2006. 281:37844–37852.

12. Liston P, Fong WG, Korneluk RG. The inhibitors of apoptosis: there is more to life than Bcl2. Oncogene. 2003. 22:8568–8580.

13. Maruyama H, Tamauchi H, Iizuka M, Nakano T. The role of NK cells in antitumor activity of dietary fucoidan from Undaria pinnatifida sporophylls (Mekabu). Planta Med. 2006. 72:1415–1417.

14. Opferman JT, Korsmeyer SJ. Apoptosis in the development and maintenance of the immune system. Nat Immunol. 2003. 4:410–415.

15. Rades D, Fehlauer F, Bajrovic A, Mahlmann B, Richter E, Alberti W. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother Oncol. 2004. 70:261–264.

16. Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995. 83:1243–1252.

17. Santini V, Giles FJ. The potential of amifostine: from cytoprotectant to therapeutic agent. Haematologica. 1999. 84:1035–1042.
18. Subramanian M, Chintalwar GJ, Chattopadhyay S. Antioxidant and radioprotective properties of an Ocimum sanctum polysaccharide. Redox Rep. 2005. 10:257–264.

19. Sweeney EA, Lortat-Jacob H, Priestley GV, Nakamoto B, Papayannopoulou T. Sulfated polysaccharides increase plasma levels of SDF-1 in monkeys and mice: involvement in mobilization of stem/progenitor cells. Blood. 2002. 99:44–51.

20. Uren AG, Pakusch M, Hawkins CJ, Puls KL, Vaux DL. Cloning and expression of apoptosis inhibitory protein homologs that function to inhibit apoptosis and/or bind tumor necrosis factor receptor-associated factors. Proc Natl Acad Sci USA. 1996. 93:4974–4978.

21. Wang ZW, Zhou JM, Huang ZS, Yang AP, Liu ZC, Xia YF, Zeng YX, Zhu XF. Aloe polysaccharides mediated radioprotective effect through the inhibition of apoptosis. J Radiat Res. 2004. 45:447–454.

22. Wrembel-Wargocka J, Jabłońska H, Chomiczewski K. Clinical use of amifostine (WR-2721) as a preparation protecting healthy tissues from the cytotoxic effects of chemotherapy and radiation therapy. Przegl Lek. 1996. 53:820–825.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download