Abstract
Purpose
To report on lessons learnt in the management of primary invasive penile cancer in a major tertiary hospital in Australia.
Materials and Methods
Medical records for all patients who underwent surgery for primary invasive penile cancer between January 2000 and January 2011 were obtained. Patient demographics, clinical status of inguinal node, cancer stage and clinical outcomes were reviewed. All patients were followed up for a minimum of 48 months postoperative unless patient deceased within the first 48 months from the time of penile cancer surgery.
Results
Over the 11-year period, a total of 23 cases of invasive penile cancer were identified. Partial penectomy was the most common form of organ preserving surgery and the majority of patients have pT1b disease. Of the 9 patients with clinically palpable inguinal nodes, 7 patients were diagnosed with pN3 disease following inguinal lymphadenectomy. The Kaplan-Meier cancer-specific survival at 72 months showed decreasing survival based on tumour stage (83% in pT1, 79% in pT2, and 64% in pT3 disease) and nodal disease (100% in node negative, 50% in superficial inguinal lymphadenopathy, and 38% in patients with deep inguinal and/or pelvic lymphadenopathy) (p=0.082). The Kaplan-Meier cancer-specific survival revealed statistically significant difference in survival outcome in patients with local recurrence vs. systemic metastasis disease (33% vs. 17%, p=0.008).
Squamous cell carcinoma of the penis is a rarely diagnosed malignancy in the developed western nations. The Cancer Council Australia reported the incidence of penile cancer at 0.6-0.9 per 100,000 population and only 82 cases of penile cancer was diagnosed in 2009 [1]. Among the known and commonly associated risk factors for penile cancer include premalignant penile lesions, phimosis, human papilloma virus transmission, chronic balanitis and inflammation as well as smoking. The presentation of penile cancer is often late due to social stigma associated with it and that penile lesion can be hidden under phimotic foreskin.
The management for penile cancer involves accurate diagnosis, grading and staging of the disease, followed by excision of the lesion. An incisional biopsy of the penile lesion provides the initial histo-pathological confirmation, while clinical detection of inguinal lymph node involvement is paramount in staging, treatment and prognosis of disease outcome [2,3]. It is a disease characterised by an essentially loco-regional spread pattern and, despite affecting a highly vascular organ, penile cancer exhibits a low frequency of hematogenous metastasis [3]. Over the past decade, considerable advances were made in the management of penile cancer in terms of diagnostic technology and treatment modalities as evidenced by volumes of scientific publication in penile cancer [4].
While there were several publications on clinical outcome in penile cancer [2,3], to our knowledge there has not been any study originating from Australia. We present the first Australian study on the clinical outcome in penile cancer management for invasive squamous cell carcinoma of the penis in a major Australian tertiary hospital.
This retrospective study was conducted following approval from our Institutional Ethics Review Board. Medical records on all consecutive patients who underwent surgery for penile cancer between January 2000 and January 2011 were obtained. Exclusion criteria for this study include patients with premalignant or noninvasive penile cancer, and nonprimary penile cancer. Patient demographics, clinical status of inguinal node, cancer stage and clinical outcomes were reviewed. Clinical staging with chest x-ray and computed tomography (CT) abdomen and pelvis was performed on all patients. All patients with clinical palpable inguinal lymphadenopathy received 6 weeks of oral cephalexin antibiotics.
The decision on the type of surgical intervention was depended on the location of the tumour and surgical description of circumcision; glansectomy; partial and total penectomy as well as that of inguinal lymph node dissection are as described in literature [2]. The minimum surgical margin clearance for penile cancer was 0.5 cm. Follow-up regimen for patient was based on published European Association of Urology penile cancer guideline at the time of review [5].
All patients were followed up for a minimum of 48 months postoperative unless patient deceased within the first 48 months from the time of penile cancer surgery. All surgical complications were recorded. Local cancer recurrence was defined as recurrence of cancer at the site of previous resection while metastasis disease was defined as systemic spread of penile cancer to other organs.
Statistical analysis was performed using chi-square test and Fisher exact test. The Kaplan-Meier survival analysis was performed to estimate cancer-specif ic survival for tumour stage, pathological node status and cancer recurrence. A significance level of p<0.05 with confidence interval 5% was used for each test. All statistical tests were performed using the SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA).
Over the 11-year period, a total of 23 cases of invasive primary penile cancer were identified and operated. Complete information was obtained in 22 patients (Table 1 for selected patients' characteristics). The mean age of patients was 64 years (39 to 87 years) with average follow-up in patients who are alive at 62.8 months (48 to 138 months). Seven patients died with an average of 18 months (2 to 36 months) from the time of surgery. The mean time from first onset of penile lesion to clinical presentation was 20.2 months (2.5 to 72 months). The majority of patients presented with ulcerating penile lesion (60%), followed by nodular (20%) and erythematous (10%) lesions. Of these men, 12 men (52%) were uncircumcised and 18 men (78%) presented with penile cancer on the foreskin (2 patients) and glans penis (16 patients). Surgical treatment for primary tumour was circumcision (2 patients), wide local excision (4 patients), glansectomy (3 patients), partial penectomy (11 patients) and total penectomy (3 patients). There was no difference in cancer stage found between circumcised or uncircumcised men. The majority of patients have pT1b (11 patients), followed by pT1a (3 patients), pT2 (5 patients), and pT3 (4 patients). There was no significant difference in surgical margin and locoregional disease (p=0.684).
A total of 9 patients (39%) had palpable inguinal nodes at clinical presentation, and 2 patients (8.7%) presented with bilateral palpable inguinal nodes. Staging studies conf irmed presence of liver metastasis in 1 patient with bilateral inguinal lymphadenopathy, and 2 patients with palpable inguinal lymphadenopathy had pelvic lymphadenopathy on CT scan. Two patients underwent lymph node dissection at the same time as excision of the primary lesion while lymph node dissection was performed in the remaining patients based on the histology of primary penile cancer within 3 months of the primary lesion resection. No sentinel node sampling was performed during primary penile surgery. All patients with palpable inguinal lympadenopathy underwent bilateral superficial inguinal lymphadenectomy (standard template in 3 patients and modified template in 6 patients), and 3 patients proceeded to pelvic lymphadenectomy. Histopathology assessment confirmed pN2 disease in 2 patients and pN3 disease in 7 patients. Three patients received adjuvant chemotherapy postoperative. Postoperative inguinal complications include lymphoedema (67%), wound dehiscence (11%), wound infection (11%), and chronic groin pain (11%). There was higher rate of complications in patients who underwent standard inguinal lymphadenectomy template and pelvic lymphadenectomy (p=0.038).
As expected, there was an association between the tumour stage and nodal disease (p<0.001). The Kaplan-Meier cancer-specific survival based on various pathological tumour stages at 72 months of follow-up were 83% in pT1, 79% in pT2, and 64% in pT3 disease (statistical significant between pT2 and pT3; p=0.038) (Fig. 1). While cancer-specific survival was similar between patients with negative superficial node vs. those with superficial inguinal lymphadenopathy at 24 months, the Kaplan-Meier cancer-specific survival in patients with node negative, superficial inguinal lymphadenopathy, and deep inguinal and/or pelvic lymphadenopathy were statistically significant at 72 months follow up (100%, 50%, and 38%, p=0.082) (Fig. 2). The Kaplan-Meier cancer-specific survival also showed statistically significant difference in survival outcome in patients with local recurrence vs. systemic metastasis disease (33% vs. 17%, p<0.008) (Fig. 3).
Penile cancer remains an uncommon malignancy in Australia [1] and many men present with advance disease in part due to social stigma and also the aggressive underlying pathological process [3]. In our series, most men waited an average of 20.2 months before presenting with an ulcerating penile lesion (60%). While the management of primary penile lesion in itself is relatively straight forward, the prognosis of patients with invasive squamous cell carcinoma or the penis requires delineation of the presence and extent of metastatic disease to regional inguinal and pelvic lymph nodes as well as secondary lesion in other organs [2].
The standard of therapy for the primary cancer is local excision with aim to preserve both urinary and/or sexual functions. While classic teaching holds that a 2-cm margin is advisable in the excision of primary penile lesion, contemporary literature suggests that the margin of resection should be based on the grade of the tumour as determined on initial biopsy. Agrawal et al. [6] showed that grades 1 and 2 tumours extend to less than 1 cm while grade 3 tumours seldom invade beyond 1.5 cm from the gross margin of the histology. Recent publication by Philippou et al. [7] reported that a surgical margin of 0.5 cm is deemed oncologically safe. In our study, we did not find any statistical difference in our tumour surgical margin and the rate of loco-regional disease (p=0.684).
The management of inguinal lymph nodes in patients with no evidence of adenopathy remains controversial as the incidence of occult metastases in patients who have no clinically palpable lymphadenopathy is reported to be around 20%-25%. Recent series have demonstrated that the rates of clinical node positivity at clinical presentation occur between 22% and 61% [8,9]. Contemporary literature supports early lymphadenectomy for clinical occult metastases as it carries potentially significant survival benefit [8,10]. While early resection of inguinal node potential appears to confer survival benefit over delayed resection [11], the morbidity of inguinal lymphadenectomy is not to be underestimated [12]. While only 2 patients in our series underwent immediate lymphadenectomy, there was no significant difference observed in the overall survival compare to men with deferred surgery within 3 months of primary penile cancer resection. When stratified to low vs. high risk patients, we did not find any difference between the pT2 and pT3 patients and locoregional disease. Similarly Hungerhuber et al. [13] reported that the risk of occult metastasis is low (8%) in low risk patients with stage T1 tumours compared to high risk patients with 75% risk of occult metastasis.
The notion of sentinel node biopsy is based on the theory of predictable sequence of lymphatic drainage and that identifying and sampling the first lymphatic drainage site to determine occult metastasis. Several techniques have been described in literature such as the use of intraoperative ultrasound and injection of blue dye or radioactive markers. However the role of sentinel node biopsy remains controversial with false negative rates estimated between 11% and 29% [14,15,16]. Unfortunately we did not advocate sentinel node biopsy in our initial workup. Nevertheless, in our series we performed inguinal lymphadenectomy in all palpable inguinal node patients.
The management of contralateral groin in patients with unilateral disease is challenging and general consensus seems to involve managing the contralateral groin based on the timing and extent of the ipsilateral groin as well as the risk stratification based on primary tumor [5]. In our series, all patients with clinical palpable nodes underwent bilateral lymphadenectomy and significant disease burden was detected in both inguinal nodes. Unfortunately this was associated with high rate of complication with two-thirds of our patients developing lymphoedema postoperative.
Disease in pelvic lymph node carries grave prognosis with 5-year survival rates of less than 20% [8,17]. The indications for pelvis lymphadenectomy are debatable because of the high morbidity of the surgery in the setting of potentially incurable disease. Patients with more than 2 positive nodes, extracapsular extension of disease and presence of high grade cancer in the inguinal node, have increased risk of pelvic node disease and pelvic surgery is advocated even if the disease is micrometastatic. Of the 9 patients who had palpable inguinal nodes in our series, staging studies confirmed presence pelvic lymphadenopathy in 2 patients at clinical presentation. One patient with high risk factor (high grade cancer, lymphovascular invasion and more than 2 positive nodes) was subsequently diagnosed with pelvic lymphadenopathy on follow up. The Kaplan-Meier cancer-specific survival in patients with node negative, superficial inguinal lymphadenopathy, and deep inguinal and/or pelvic lymphadenopathy were statistically significant at 72 months follow up (100%, 50%, and 38%, p=0.082).
Our study has several limitations that should be highlighted. This is a retrospective analysis of a small historical series of patients treated at different times in which the understanding of the disease has changed considerably and can affect the treatment protocol in our series over the 11-year study period. Nevertheless, the survival analysis of our cohort based on primary tumour stage, lymph node involvement and metastasis is similar to contemporary literature. Our study demonstrated that penile sparing surgery is not associated with risk of local recurrence provided that there is a minimum of 0.5-cm surgical cancer clearance. While early lymphadenectomy carries better prognosis especially in patients with intermediate or high risk disease, the survival outcome did not differ significantly provided that surgery was carried out within 3 months of the primary lesion in our cohort. The survival benefit of inguinal lymphadenectomy probably outweighs the morbidity of surgery but patients should be counselled on grave prognosis if the nodes are positive. It is likely that there should be a concentration of penile cancer surgery to major tertiary hospital since this disease is rare and the management of penile cancer requires high expertise. The future of penile cancer management should focus on disease prevention such as penile cancer vaccines and other molecular targeting agents. Until such time, larger, multinational prospective trials on controversial issues need to be conducted to improve our management in penile cancer.
The management of patients with invasive squamous cell carcinoma of the penis requires early diagnosis and risk stratification. Penile preserving strategies, accurate clinical staging and management of lymph node disease are important consideration to take into account. The presence of high risk features such as tumour stage, lymph node involvement and distant metastasis carries poor prognosis.
Figures and Tables
Fig. 1
The Kaplan-Meier cancer-specific survival in patients based on various pathological tumour stages at 72 months were 83% in pT1, 79% in pT2, and 64% in pT3 disease (statistical significant between pT2 and pT3; p=0.038).
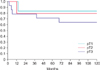
Fig. 2
The Kaplan-Meier cancer-specific survival in patients with node negative, superficial inguinal lymphadenopathy, and deep inguinal and/or pelvic lymphadenopathy were 100%, 50%, and 38% at 72 months (p=0.082).

Fig. 3
The Kaplan-Meier cancer-specific survival in patients with no recurrence, local recurrence and systemic metastasis were 100%, 33%, and 17% at 72 months (p=0.008).

Table 1
Summary of selected patients' characteristics

References
1. Australian Institute of Health and Welfare (AIHW) & Australasian Association of Cancer Registries 2012. Cancer in Australia: an overview 2012. Cancer series no. 74. Cat. no. CAN 70. Canberra: AIHW;2012.
2. Deem S, Keane T, Bhavsar R, El-Zawahary A, Savage S. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011; 108:1378–1392.
3. Caso JR, Rodriguez AR, Correa J, Spiess PE. Update in the management of penile cancer. Int Braz J Urol. 2009; 35:406–415.
4. Pompeo AC, Heyns CF, Abrams P. Penile cancer: international consultation on penile cancer. Cape Town: CTP Book Printers;2009.
5. Solsona E, Algaba F, Horenblas S, Pizzocaro G, Windahl T. European Association of Urology. EAU guidelines on penile cancer. Eur Urol. 2004; 46:1–8.
6. Agrawal A, Pai D, Ananthakrishnan N, Smile SR, Ratnakar C. The histological extent of the local spread of carcinoma of the penis and its therapeutic implications. BJU Int. 2000; 85:299–301.
7. Philippou P, Shabbir M, Malone P, Nigam R, Muneer A, Ralph DJ, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012; 188:803–808.
8. Lont AP, Kroon BK, Gallee MP, van Tinteren H, Moonen LM, Horenblas S. Pelvic lymph node dissection for penile carcinoma: extent of inguinal lymph node involvement as an indicator for pelvic lymph node involvement and survival. J Urol. 2007; 177:947–952.
9. Guimaraes GC, Lopes A, Campos RS, Zequi Sde C, Leal ML, Carvalho AL, et al. Front pattern of invasion in squamous cell carcinoma of the penis: new prognostic factor for predicting risk of lymph node metastases. Urology. 2006; 68:148–153.
10. Leveridge M, Siemens DR, Morash C. What next? Managing lymph nodes in men with penile cancer. Can Urol Assoc J. 2008; 2:525–531.
11. Kroon BK, Horenblas S, Lont AP, Tanis PJ, Gallee MP, Nieweg OE. Patients with penile carcinoma benefit from immediate resection of clinically occult lymph node metastases. J Urol. 2005; 173:816–819.
12. Bouchot O, Rigaud J, Maillet F, Hetet JF, Karam G. Morbidity of inguinal lymphadenectomy for invasive penile carcinoma. Eur Urol. 2004; 45:761–765.
13. Hungerhuber E, Schlenker B, Karl A, Frimberger D, Rothenberger KH, Stief CG, et al. Risk stratification in penile carcinoma: 25-year experience with surgical inguinal lymph node staging. Urology. 2006; 68:621–625.
14. Tanis PJ, Lont AP, Meinhardt W, Olmos RA, Nieweg OE, Horenblas S. Dynamic sentinel node biopsy for penile cancer: reliability of a staging technique. J Urol. 2002; 168:76–80.
15. Spiess PE, Izawa JI, Bassett R, Kedar D, Busby JE, Wong F, et al. Preoperative lymphoscintigraphy and dynamic sentinel node biopsy for staging penile cancer: results with pathological correlation. J Urol. 2007; 177:2157–2161.
16. Hadway P, Smith Y, Corbishley C, Heenan S, Watkin NA. Evaluation of dynamic lymphoscintigraphy and sentinel lymphnode biopsy for detecting occult metastases in patients with penile squamous cell carcinoma. BJU Int. 2007; 100:561–565.
17. Culkin DJ, Beer TM. Advanced penile carcinoma. J Urol. 2003; 170(2 Pt 1):359–365.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download