Abstract
Renal cell carcinoma associated with fused ectopic kidneys has rarely been reported in the literature. Here we report the first case of robot-assisted heminephrectomy for chromophobe renal cell carcinoma in an L-shaped fused ectopic kidney. The present case report highlights the importance of three-dimensional vision and enhanced maneuverability with the EndoWrist technology of the robotic surgical system for precise dissection. This report also highlights the importance of preoperative contrast-enhanced computed tomography with three-dimensional arterial reconstruction for surgical planning.
Renal cell carcinoma associated with a fused ectopic kidney has rarely been reported in the literature. We report the first case of robot-assisted heminephrectomy for chromophobe renal cell carcinoma in a fused ectopic kidney in a 55-year-old woman. This report highlights the importance of preoperative contrast-enhanced computed tomography (CECT) with arterial three-dimensional (3D) reconstruction for preoperative surgical planning, the advantages of the 3D vision of the robot with EndoWrist technology (Intuitive Surgical Inc., Sunnyvale, CA, USA) for careful dissection, and the technical difficulties encountered when such large tumors are tackled with minimally invasive surgery.
A 55-year-old woman presented with dull aching pain on the right side of her abdomen. Abdominal examination revealed an ill-defined lump in the right lumbar area extending to the hypogastrium and iliac fossa. Ultrasound revealed a right-sided renal mass with an empty left renal fossa with an ectopic kidney at the L4 level. Dedicated triphasic CECT of the abdomen was performed for preoperative surgical planning. The CT revealed crossed fused renal ectopia on the right side (Fig. 1). The right renal moiety was lying opposite the L1-L4 vertebrae. The left moiety was seen lying horizontally opposite the L4-L5 vertebrae with its pelvis lying anteriorly and forming an L-shaped crossed fused ectopia. A 15-cm lobulated, minimally enhancing mass lesion was noticed to arise from the anteromedial aspect of the orthotopic kidney in the lower and interpolar region. The mass lesion was infiltrating the renal sinus fat and the upper pole region of the left moiety with ill-defined fat planes with the second part of the duodenum and liver.
The arterial phase of the CECT revealed three renal arteries arising from the abdominal aorta. The proximal renal artery was seen arising at the L1 level at the 9 o'clock position and was supplying the right renal moiety along with the mass lesion. The mid renal artery was arising at the upper border of the L3 level at the 10 o'clock position and was supplying the mass lesion along with the ectopic moiety. The distal renal artery originated at the mid L3 level and was supplying the ectopic moiety. Two renal veins were at the L1-L2 level draining the orthotopic moiety along with the mass lesion. Another renal vein at the lower border of the L3 level was draining the mass lesion and the left renal moiety into the inferior vena cava. The left moiety was also drained by another renal vein into the common iliac vein at the L4 level. Both renal moieties showed normal contrast excretion. The right ureter was seen coursing along the medial margin of the mass lesion, creating a plane between the mass and the left renal moiety. The left ureter was crossing the midline and was draining into the left vesicoureteric junction. No significant regional lymphadenopathy was present. The patient's serum creatinine was 1.01 mg/dL.
After the preanesthetic checkup, the patient underwent robot-assisted radical heminephrectomy on the right side under general anesthesia (Fig. 2). The patient was placed in the left lateral decubitus position and was well secured with adequate pressure padding. Pneumoperitoneum was created by using a Veress needle through an umbilical incision. A 12-mm trocar was inserted at the umbilicus and a 30-degree scope was introduced. Two 8-mm robotic working ports were placed at the lateral border of the rectus, one between the xiphoid process and the umbilicus and the other between the umbilicus and the pubic symphysis. Another 12-mm assistant port was placed in a midline supraumbilical location. Docking of the robot was done. The right colon was mobilized medially. By use of blunt and sharp dissection, the renal mass was mobilized at the superior pole followed by mobilization at the inferior pole with identification of the right ureter between the renal mass and the ectopic kidney where it was clipped and divided. On the basis of the preoperative cross-sectional imaging, tumor-feeding vessels were clipped and divided. The lower limit of the tumor was meticulously dissected off from the ectopic moiety with preservation of its vasculature. The parenchyma at the isthmus was quite thin, and bleeding was controlled by electrocautery. The collecting system was not opened. Thus, no formal repair of the thin raw area was needed. In an attempt to avoid iatrogenic injury to the mesenteric vessels and blood vessels supplying the healthy ectopic moiety, dissection was kept close to the tumor. The large size of the tumor was impairing vision and light focusing. While dissecting superiorly and posteriorly, the plane of dissection went through the upper polar normal parenchyma of the orthotopic kidney, which was supplied by a separate vascular twig, which kept on oozing until the kidney was completely mobilized. Identification, clipping, and division of this vascular pedicle achieved hemostasis. The large tumor size hindered its retrieval with an indigenous specimen delivery bag. For specimen delivery and hemostasis, an anterior subcoastal incision was made. A 22-Fr abdominal drain was placed. Wounds were sutured and the patient was shifted to recovery after extubation.
The total operating time was 120 minutes (16 minutes for port placement and 2 minutes for docking). Blood loss was 600 mL. Gross examination of the specimen revealed an 18 cm×12 cm mass arising from the right kidney with areas of hemorrhage and necrosis. The patient was ambulatory by the second postoperative day. Final histopathology revealed chromophobe renal cell carcinoma with negative surgical resection margins.
The reported incidence of crossed fused ectopia is 1 in 1,000 live births [1]. Ninety percent of crossed ectopic kidneys are fused with their ipsilateral mate [1]. The male-to-female incidence ratio is 2:1 and crossed fused ectopia is three times as common on the left side as on the right side [23]. Pathogenesis of crossed fused ectopia involves abnormal ureteric bud development, malalignment, and abnormal rotation of the caudal end of the fetus; genetic and teratogenic factors; or abnormally placed umbilical arteries [1]. Fusion can occur at any stage of ascent and rotation. An L-shaped or tandem kidney occurs when the crossed kidney lies in a transverse plane at the time of fusion with the lower pole of the orthotopic kidney. The ectopic kidney lies against the L4 vertebra [1]. Usually, the native renal units are normal. Abnormalities in the ectopic moiety involve cystic dysplasia, obstruction, reflux, infection, urolithiasis, twisting, or volvulus [4].
Association with malignancy is rare and the reported incidence of malignancy is no greater than in the normal population [5]. Only seven cases of crossed fused ectopia with renal malignancy have been reported [4]. All of the cases were renal cell carcinoma except for one case of transitional cell carcinoma [6].
Regardless of the type of ectopia and fusion, the vascular supply of both the orthotopic and the ectopic moiety is highly variable and unpredictable. One or more renal arteries arising at a variable level from the aorta or common iliac vessels may supply the renal units. As noted in our case, preoperative CECT with 3D reconstructed imaging helps in surgical planning in such cases.
Romero et al. [4] reported the first case of laparoscopic heminephrectomy for renal cell carcinoma in crossed fused ectopia in 2007. This is the first case report describing robot-assisted radical nephrectomy for chromophobe renal cell carcinoma with fused crossed ectopia. The 3D vision and the EndoWrist technology aided in meticulous dissection of the renal mass from the normal ectopic moiety and identification of the multiple vascular pedicles supplying the tumor. The large tumor size, the multiple anomalous vessels, and the need to preserve the ectopic moiety were the major concerns. To avoid inadvertent injury to the mesenteric vessels and vessels supplying the ectopic moiety, dissection was kept close to the tumor mass. However, during posterior dissection, the plane of dissection went through the upper polar normal renal parenchyma of the orthotopic kidney, which was not in the line of vision owing to the large tumor size. Such problems do occur while mobilizing such large renal masses. The major advantage was identification of the vascular pedicles feeding the tumor along with identification of the plane between the renal mass and the ectopic moiety. Robotic assistance also resulted in a shorter incision length and earlier convalescence and return to work.
This case report highlights the advantages of CECT with arterial 3D reconstruction for preoperative surgical planning, the advantages of the 3D vision of the robot for careful dissection, and the technical difficulties encountered when such large tumors are tackled with minimally invasive surgery.
Figures and Tables
Fig. 1
(A-C) Axial, coronal and oblique sections show tumor arising from the orthotopic moiety. Arrow marks the junction between the ectopic and the orthotopic moiety. (D) Axial section shows horizontally lying pelvic ectopic kidney. (E) Three-dimensional angiogram demonstrating the origin and course of multiple renal arteries. (F) Delayed phase image depicting the pelvi-calyceal system and ureteric course of both moieties. The ureter of orthotopic moiety is coursing between tumor and ectopic moiety.
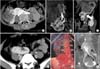
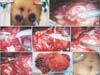
Fig. 2
Port placement (A); tumor being dissected (B); vascular pedicles being clipped and divided (C); ureter of orthotopic moiety dissected (D); plane of dissection between tumor mass and ectopic moiety (*) (E); tumor mass dissected all-around (F); gross specimen (G); postoperative wound (H). R, robotic arms; C, camera port, A, assistant port.
References
1. Shapiro E, Bauer SB, Chow JS. Anomalies of the upper urinary tract. In : Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh urology. 10th ed. Philadelphia: Saunders;2012. p. 3140–3145.
2. Stanley KE, Winfield HN, Donovan JF, Fallon B. Laparoscopic nephrectomy in crossed fused renal ectopia. Urology. 1993; 42:375–378.
3. Felzenberg J, Nasrallah PF. Crossed renal ectopia without fusion associated with hydronephrosis in an infant. Urology. 1991; 38:450–452.
4. Romero FR, Chan DY, Muntener M, Bagga HS, Brito FA, Kavoussi LR. Laparoscopic heminephrectomy for renal cell carcinoma in cross-fused ectopic kidney. Urology. 2007; 69:779.e11–779.e13.
5. Stimac G, Dimanovski J, Ruzic B, Spajic B, Kraus O. Tumors in kidney fusion anomalies--report of five cases and review of the literature. Scand J Urol Nephrol. 2004; 38:485–489.
6. Gur U, Yossepowitch O, Baniel J. Transitional cell carcinoma in a fused crossed ectopic kidney. Urology. 2003; 62:748.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download