Abstract
The therapeutic effects and side effects of androgen deprivation therapy (ADT), which is a main treatment method for metastatic prostate cancer, are well known, but the metabolic effects have only recently been studied. This review describes the effects of ADT on body habitus, insulin resistance, lipid profiles, diabetes, metabolic syndrome, and cardiovascular morbidity and mortality. The review was done by using KoreaMed and PubMed to search the medical literature related to prostate cancer, ADT, body habitus, lipid profile, diabetes, insulin resistance, metabolic syndrome, and cardiovascular disease. ADT increases fat mass and decreases lean body mass. Fat mostly accumulates in the subcutaneous area. ADT increases total cholesterol, triglycerides, and high-density lipoprotein, as well as the risk for insulin resistance and diabetes. ADT also increases the risk for cardiovascular events, but insufficient evidence is available for a correlation with mortality. ADT changes body habitus and lipid profiles and has different characteristics than those of classic metabolic syndrome, but it is related to insulin resistance and diabetes. ADT increases the risk for cardiovascular events. No consistent guidelines have been proposed for treating the metabolic effects of ADT, but the generally recommended treatment methods for lowering the risk of diabetes and cardiovascular disease should be fully understood. Additional studies are necessary.
Prostate cancer is the most commonly diagnosed cancer in men and has the sixth highest related mortality [1,2,3,4]. Prostate cancer-related mortality is decreasing worldwide as a result of progress in prostate cancer treatment methods. Androgen deprivation therapy (ADT) is one of the main methods of treating prostate cancer. ADT uses either bilateral orchiectomy or gonadotropin-releasing hormone (GnRH) agonists [5]. GnRH agonists inhibit production of endogenous testosterone but have side effects, such as loss of libido, hot flashes, osteoporosis, fatigue, loss of lean body mass, anemia, and gynecomastia [6,7]. ADT causes metabolic effects and thus is related to changes in body habitus and lipid profiles and an increased risk for insulin resistance, diabetes, and metabolic syndrome [8,9,10]. Some studies have reported that ADT is also related to cardiovascular morbidity and mortality [11,12,13,14]. There is a schematic diagram of adverse and metabolic effects of ADT (Fig. 1). This review describes the metabolic effects of ADT.
Androgens increase lean body mass and decrease fat mass [15], whereas ADT increases fat mass and decreases lean body mass [16,17]. A prospective study was conducted on changes in body composition caused by 1 year of GnRH agonist use in 40 men with locally advanced nonmetastatic prostate cancer. The weight increase after 1 year was 2.4%±0.8% (p=0.005), percentage body fat mass increased 9.4%±1.7% (p<0.001), and the percentage of lean body mass decreased 2.7%±0.5% (p<0.001) [17]. Similarly, a study of 79 patients with nonmetastatic prostate cancer showed a weight increase of 1.8%±0.5% (p<0.001) during 1 year of ADT. Their percentage fat mass increased by 11.0%±1.7%, and their percentage lean mass decreased by 3.8%±0.6% [18].
Fat accumulation is mostly subcutaneous, and intra-abdominal fat does not change significantly during GnRH agonist treatment. According to a prospective study by Smith et al. [17], cross-sectional subcutaneous fat area increased significantly by 11.1%±3.4% (p=0.003) during 1 year of ADT, but intra-abdominal fat area did not change (p=0.94).
ADT-induced body composition changes occur during the early stage of treatment. Fat mass and circulating insulin increased in 22 patients after 3 months of ADT given prospectively [19]. Another study reported that body fat mass increased by 4.3%±1.3% (p=0.002) when androgen blockade was combined with a GnRH agonist and bicalutamide over 12 weeks in 25 patients [20]. Lee et al. [21] reported on 65 patients who received ADT for 12 months. They found that the increase in fat mass and lean body mass mostly occurred during the early stage of GnRH agonist therapy and that the body composition changes decreased if the treatment period was extended.
GnRH agonists cause changes in serum lipids. Many studies have reported that increased lipid levels occur mainly in total cholesterol, triglycerides, and high densitylipoprotein (HDL) [17,22,23]. Eri et al. [23] administered a GnRH agonist to 26 patients for 24 weeks and found significant increases in total cholesterol (10.6%), HDL (8.2%), and triglycerides (26.9%) (p=0.003, p=0.052, and p=0.050, respectively), but low-density lipoprotein (LDL) did not change. Another study reported increases in total cholesterol (9.0%), HDL (11.3%), triglycerides (26.5%), and LDL (7.3%) levels when a GnRH agonist was given for 1 year to 40 patients with prostate cancer [17]. According to a study by Torimoto et al. [24], significant increases in total cholesterol and LDL were observed at baseline and at 3, 6, 9, and 12 months in 39 patients with prostate cancer treated with ADT [24]. Salvador et al. [25] assessed and observed changes in lipid profiles caused by ADT in 33 patients with locally advanced or metastatic prostate cancer, both prospectively before ADT and at 6 and 12 months after ADT. Total cholesterol increased from 210 mg/dL at baseline to 227 mg/dL at 6 months (p<0.05), and LDL increased from 132 mg/dL to 148 mg/dL during the same period (p<0.05). HDL and triglycerides did not change. No significant differences were observed in any component at 12 months [25].
There is no consensus on changes in lipid profiles after ADT, but many studies report increased total cholesterol, triglycerides, and HDL levels.
Insulin resistance is a metabolic malfunction accompanied by diabetes, prediabetes, and obesity, and it is an independent risk factor for cardiovascular disease [26,27]. Insulin resistance caused by a GnRH agonist begins in the early stage of treatment and increases risks for diabetes and coronary artery disease [28]. GnRH agonists increase fasting insulin levels [19,22] and decrease insulin sensitivity in patients with prostate cancer [20,29].
Smith et al. [29] reported that serum glucose measurements from an oral glucose tolerance test (OGTT) given before prostate cancer treatment did not differ from those after 12 weeks of a combined androgen blockade, but a significant difference in insulin levels was observed before and after treatment [29]. They also reported prospectively on 22 patients undergoing GnRH agonist treatment for prostate cancer and found that median serum insulin levels increased gradually from 11.8 mU/L (range, 5.6-49.1 mU/L) before treatment to 15.1 mU/L (range, 7.3-83.2 mU/L) after 1 month of treatment and to 19.3 mU/L (range, 0-85.0 mU/L) after 3 months of GnRH agonist treatment [19]. Dockery et al. [22] studied 16 patients receiving GnRH agonist treatment and reported that serum insulin levels increased from 6.89±4.84 mU/L before treatment to 11.34±8.16 mU/L by 3 months of treatment.
Obesity and insulin resistance are closely related to type 2 diabetes [30]. Derweesh et al. [31] conducted a retrospective study of 396 patients who received ADT; 36 patients (11.3%) were newly diagnosed with diabetes during follow-up (median follow-up duration, 60.1 months). Fifteen patients (19.5%) showed an increase of 10% or higher in glycosylated hemoglobin among the 77 patients who were already diagnosed with diabetes; 22 patients (28.6%) had increases in fasting glucose levels of 10% or higher [31].
Keating et al. [28] conducted a large population-based study using the surveillance, epidemiology, and end results (SEER) and Medicare databases to assess the correlation between ADT and newly diagnose diabetes. The study was conducted on 73,196 male patients who were ≥66 years old and who had been diagnosed with prostate cancer. They reported that the use of a GnRH agonist increased the risk for incident diabetes (hazard ratio [HR], 1.44; p<0.001), and a bilateral orchiectomy increased the risk of diabetes (HR, 1.34; p<0.001) [28]. These authors conducted an additional study on 37,443 male patients of all ages who were diagnosed with prostate cancer; 14,597 of those patients (39%) received ADT [32]. They reported that GnRH agonist treatment was significantly related to an increased risk of incident diabetes (HR, 1.28; 95% confidence interval [CI], 1.19-1.38) [32].
Alibhai et al. [33] analyzed 19,079 patients who had at least 6 months of ADT treatment or underwent a bilateral orchiectomy after receiving a prostate cancer diagnosis by matching those patients one-on-one with non-ADT patients. They reported that ADT increased the risk for diabetes (HR, 1.24; 95% CI, 1.15-1.35; p<0.05), and ADT administration for 24 months or longer was related to the onset of diabetes more than was ADT treatment for less than three months. These authors concluded that the duration of ADT increases the risk for diabetes [33].
Metabolic syndrome has various characteristics, including increased body fat, waist circumference, blood pressure, and triglycerides, as well as decreased HDL. Metabolic syndrome is a diagnostic cluster related to insulin resistance and cardiovascular disease risk factors.
Braga-Basaria et al. [34] conducted a cross-sectional study to evaluate the prevalence of metabolic syndrome in 58 patients who received long-term ADT. The prevalence of metabolic syndrome was 55% in the ADT group, which had at least 12 months of ADT, and was higher than that in the non-ADT and control groups (p<0.01 and p=0.03, respectively). The prevalence rates of abdominal obesity and hyperglycemia were higher in the ADT group, and triglycerides also increased significantly more in the ADT group (p=0.02) than in the control group, but no differences in the prevalence rates of hypertension or LDL were observed [34].
Various studies have reported that ADT increases fat and triglycerides and decreases insulin sensitivity [34,35]. However, metabolic alterations caused by ADT have characteristics different from those of classic metabolic syndrome, as ADT increases HDL and subcutaneous fat rather than visceral abdominal fat [17,35]. Although classic metabolic syndrome is related to a decrease in adiponectin [36] and an increase in C-reactive protein [37], ADT increases adiponectin and does not change C-reactive protein [29,35]. We compare and summarize the metabolic effects caused by classic metabolic syndrome and ADT in Table 1.
Many studies have evaluated whether ADT increases the risk for cardiovascular disease, as it does affect body habitus, lipid profiles, insulin resistance, type 2 diabetes, and metabolic syndrome. Keating et al. [28] assessed ADT and cardiovascular risk in 73,196 people in the SEER and Medicare database and found that men who received GnRH agonist treatment had an increased rate of onset of coronary heart disease (HR, 1.16; p<0.001), myocardial infarction (HR, 1.11; p=0.03), and sudden cardiac death (HR, 1.16; p=0.004). In addition, ADT increased serious cardiovascular morbidity by 20% in a retrospective analysis of 23,000 patients with prostate cancer [11].
In the Radiation Therapy Oncology Group (RTOG) 85-31 trial [38], more than 900 patients with T3 tumors or lymph node-involved prostate cancer were compared in a radiotherapy (RT) group and an RT-combined-withindefinite-ADT group. Cardiovascular mortality was found to be 8.4% when RT was combined with indefinite ADT and 11.4% when only RT was administered (p=0.17) [12,39]. ADT did not significantly increase cardiovascular mortality in the RTOG 86-10 [38] and RTOG 92-02 [40] trials.
The European Organization f or Research and Treatment of Cancer Trial 30891 compared a group in which ADT was applied immediately and another group in which ADT was applied only after symptoms arose [41]. A total of 985 patients with prostate cancer who were not suitable for local treatment and the latter group had a median time of 7 years from randomization to ADT. Cardiovascular mortality was 17.9% for the immediate-ADT group and 19.7% for the group in which ADT was applied only after symptoms appeared (p>0.05) [41].
Nguyen et al. [42] conducted a meta-analysis on randomized controlled trials (RCTs) to investigate the correlation between ADT and cardiovascular disease in patients with prostate cancer. From the results of eight RCTs involving 4,141 men, no significant difference in cardiovascular death was observed between ADT groups and non-ADT groups (incidence, 11.0%; 95% CI, 8.3-14.5 vs. 11.2%; 95% CI, 8.3-15.0; relative risk [RR], 0.93; 95% CI, 0.79-1.10; p=0.41), and no correlation was found between ADT and cardiovascular death in a long-term trial with a minimum of 3 years of ADT treatment (11.5%; 95% CI, 8.1-16.0 vs. 11.5%; 95% CI, 7.5-17.3; RR, 0.91; 95% CI, 0.75-1.10; p=0.34). No correlation was observed between cardiovascular death and a short-term trial with less than 6 months of ADT (10.5%; 95% CI, 6.3-17.0 vs. 10.3%; 95% CI, 8.2-13.0; RR, 1.00; 95% CI, 0.7-1.37; p=0.99).
Thus, ADT appears to increase morbidity from cardiovascular disease, but no clear evidence has been presented on whether it increases cardiovascular mortality.
GnRH agonists are widely used to treat prostate cancer. GnRH agonists cause metabolic effects, such as changes in body habitus, insulin resistance, and lipid profiles, and also increase the risk for diabetes and cardiovascular events. Therefore, it is important to manage such metabolic changes. Despite the absence of high-level evidence for outcome benefits specific to men with prostate cancer receiving ADT, Grossman and Zajac [43] summarized the assessment and management of metabolic and cardiovascular health in men with prostate cancer who were receiving ADT. First, a metabolic risk assessment before beginning ADT treatment should include body mass index, waist circumference, blood pressure, fasting blood glucose, OGTT (if fasting glucose is 100-125 mg/dL), and a fasting lipid profile. Second, the metabolic assessment should be repeated at 6 and 12 months, with subsequent testing. Third, intensive intervention should be instituted to prevent weight gain and worsening of insulin resistance. Fourth, management should include reducing cardiovascular risk factors, particularly smoking cessation. Blood pressure should be <130/80 mmHg, and lipid targets should follow the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP-III) treatment guidelines.
The American Diabetes Association specif ically recommends a 10% weight reduction and at least 150 minutes of exercise per week for patients with impaired glucose tolerance or impaired fasting glucose [44]. Active treatment of hyperlipidemia is effective for treating cardiovascular disease and can be applied to patients receiving ADT. According to NCEP ATP-III, the two goals of insulin resistance treatment are fixing underlying causes if possible and treating cardiovascular risk factors if issues continue after changing lifestyle [45,46]. In addition, NCEP ATP-III recommends using statins as firstline medication when lifestyle intervention does not lead to the goal [46].
As there are no consistent guidelines for treating the metabolic effects of ADT, these effects should be fully understood, and treatment should be performed according to the recommended guidelines to lower the risk of diabetes and cardiovascular disease. We believe that additional studies and treatment guidelines are necessary to assess the metabolic effects of ADT.
Figures and Tables
Fig. 1
Adverse effects of androgen deprivation therapy (ADT). Inside the dotted line represents metabolic effects of ADT. DM, diabetes mellitus.
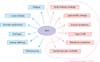
Table 1
Comparison of classic metabolic syndrome and the metabolic effects of ADT

References
1. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011; 61:212–236.
2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127:2893–2917.
3. Baade PD, Youlden DR, Krnjacki LJ. International epidemiology of prostate cancer: geographical distribution and secular trends. Mol Nutr Food Res. 2009; 53:171–184.
4. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
5. Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011; 59:572–583.
6. Jeong SJ, Kwak C, Lee SE. Therapeutic effect of maximal androgen blockade in metastatic prostate cancer. Korean J Urol. 2001; 42:642–649.
7. Flaig TW, Glode LM. Management of the side effects of androgen deprivation therapy in men with prostate cancer. Expert Opin Pharmacother. 2008; 9:2829–2841.
8. Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol. 2013; 189:1 Suppl. S34–S42.
9. Nobes JP, Langley SE, Laing RW. Metabolic syndrome and prostate cancer: a review. Clin Oncol (R Coll Radiol). 2009; 21:183–191.
10. Leahy Y. Risk of metabolic syndrome, cardiovascular disease, and diabetes in androgen deprivation therapy. Clin J Oncol Nurs. 2008; 12:771–776.
11. Saigal CS, Gore JL, Krupski TL, Hanley J, Schonlau M, Litwin MS, et al. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007; 110:1493–1500.
12. Efstathiou JA, Bae K, Shipley WU, Hanks GE, Pilepich MV, Sandler HM, et al. Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85-31. J Clin Oncol. 2009; 27:92–99.
13. Van Poppel H, Tombal B. Cardiovascular risk during hormonal treatment in patients with prostate cancer. Cancer Manag Res. 2011; 3:49–55.
14. Roayaei M, Ghasemi S. Effect of androgen deprivation therapy on cardiovascular risk factors in prostate cancer. J Res Med Sci. 2013; 18:580–582.
15. Vermeulen A, Goemaere S, Kaufman JM. Testosterone, body composition and aging. J Endocrinol Invest. 1999; 22:5 Suppl. 110–116.
16. Berruti A, Dogliotti L, Terrone C, Cerutti S, Isaia G, Tarabuzzi R, et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002; 167:2361–2367.
17. Smith MR, Finkelstein JS, McGovern FJ, Zietman AL, Fallon MA, Schoenfeld DA, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002; 87:599–603.
18. Smith MR. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology. 2004; 63:742–745.
19. Smith JC, Bennett S, Evans LM, Kynaston HG, Parmar M, Mason MD, et al. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001; 86:4261–4267.
20. Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006; 91:1305–1308.
21. Lee H, McGovern K, Finkelstein JS, Smith MR. Changes in bone mineral density and body composition during initial and long-term gonadotropin-releasing hormone agonist treatment for prostate carcinoma. Cancer. 2005; 104:1633–1637.
22. Dockery F, Bulpitt CJ, Agarwal S, Donaldson M, Rajkumar C. Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci (Lond). 2003; 104:195–201.
23. Eri LM, Urdal P, Bechensteen AG. Effects of the luteinizing hormone-releasing hormone agonist leuprolide on lipoproteins, fibrinogen and plasminogen activator inhibitor in patients with benign prostatic hyperplasia. J Urol. 1995; 154:100–104.
24. Torimoto K, Samma S, Kagebayashi Y, Chihara Y, Tanaka N, Hirayama A, et al. The effects of androgen deprivation therapy on lipid metabolism and body composition in Japanese patients with prostate cancer. Jpn J Clin Oncol. 2011; 41:577–581.
25. Salvador C, Planas J, Agreda F, Placer J, Trilla E, Lopez MA, et al. Analysis of the lipid profile and atherogenic risk during androgen deprivation therapy in prostate cancer patients. Urol Int. 2013; 90:41–44.
26. Despres JP, Lamarche B, Mauriège P, Cantin B, Dagenais GR, Moorjani S, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996; 334:952–957.
27. Pyorala M, Miettinen H, Laakso M, Pyorala K. Hyperinsulinemia predicts coronary heart disease risk in healthy middleaged men: the 22-year follow-up results of the Helsinki Policemen Study. Circulation. 1998; 98:398–404.
28. Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006; 24:4448–4456.
29. Smith MR, Lee H, Fallon MA, Nathan DM. Adipocytokines, obesity, and insulin resistance during combined androgen blockade for prostate cancer. Urology. 2008; 71:318–322.
30. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014; 37:Suppl 1. S81–S90.
31. Derweesh IH, Diblasio CJ, Kincade MC, Malcolm JB, Lamar KD, Patterson AL, et al. Risk of new-onset diabetes mellitus and worsening glycaemic variables for established diabetes in men undergoing androgen-deprivation therapy for prostate cancer. BJU Int. 2007; 100:1060–1065.
32. Keating NL, OMalley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010; 102:39–46.
33. Alibhai SM, Duong-Hua M, Sutradhar R, Fleshner NE, Warde P, Cheung AM, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009; 27:3452–3458.
34. Braga-Basaria M, Dobs AS, Muller DC, Carducci MA, John M, Egan J, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006; 24:3979–3983.
35. Smith MR, Lee H, McGovern F, Fallon MA, Goode M, Zietman AL, et al. Metabolic changes during gonadotropin-releasing hormone agonist therapy for prostate cancer: differences from the classic metabolic syndrome. Cancer. 2008; 112:2188–2194.
36. Trujillo ME, Scherer PE. Adiponectin: journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005; 257:167–175.
37. Haffner SM. The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol. 2006; 97(2A):3A–11A.
38. Roach M 3rd, Bae K, Speight J, Wolkov HB, Rubin P, Lee RJ, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol. 2008; 26:585–591.
39. Pilepich MV, Winter K, Lawton CA, Krisch RE, Wolkov HB, Movsas B, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma: long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005; 61:1285–1290.
40. Efstathiou JA, Bae K, Shipley WU, Hanks GE, Pilepich MV, Sandler HM, et al. Cardiovascular mortality and duration of androgen deprivation for locally advanced prostate cancer: analysis of RTOG 92-02. Eur Urol. 2008; 54:816–823.
41. Studer UE, Whelan P, Albrecht W, Casselman J, de Reijke T, Hauri D, et al. Immediate or deferred androgen deprivation for patients with prostate cancer not suitable for local treatment with curative intent: European Organisation for Research and Treatment of Cancer (EORTC) Trial 30891. J Clin Oncol. 2006; 24:1868–1876.
42. Nguyen PL, Je Y, Schutz FA, Hoffman KE, Hu JC, Parekh A, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA. 2011; 306:2359–2366.
43. Grossmann M, Zajac JD. Management of side effects of androgen deprivation therapy. Endocrinol Metab Clin North Am. 2011; 40:655–671.
44. American Diabetes Association. Standards of medical care in diabetes--2008. Diabetes Care. 2008; 31:Suppl 1. S12–S54.
45. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–2497.
46. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002; 106:3143–3421.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download