Abstract
Purpose
Kidney stones (nephrolithiasis) are a widespread disease. Thus, blocking stone formation and finding new therapeutic methods is an important area of study. Diosmin (a major component of the bile) is known to have antioxidant as well as renoprotective effects. The present investigation aimed to evaluate the effect of diosmin on renal tissue protection in rats with ethylene glycol-induced nephrolithiasis.
Materials and Methods
The rats were randomly divided into three groups. Group one (control) did not receive any treatments. In groups two and three, nephrolithiasis was induced by 2.5% (V/V) ethylene glycol + 2.5% (W/V) ammonium chloride (2 mL/d). The second and the third groups received distilled water or diosmin (80 mg/kg/d) by gavage for 21 days.
Results
Stereological estimation of the renal structures revealed that the average volume of calcium oxalate (CaOx) in the nephrolithiasis+diosmin rats was -63% less than in the rats with untreated nephrolithiasis (p<0.01). The volume of the glomeruli, proximal and distal convoluted tubules, Henle's loop, collecting ducts, and vessels was reduced -32% to 58% after the induction of nephrolithiasis (p<0.001). In the nephrolithiasis+diosmin rats, on average, -70% to 96% of the glomeruli, proximal convoluted tubules, Henle's loop collecting ducts, and vessels remained intact (p<0.01). Degeneration of the cortical tissue was 5-fold that of the medulla. In the nephrolithiasis+diosmin rats, degeneration in the renal cortical tissue and medulla was reduced -70% and 44%, respectively, compared with that in the untreated nephrolithiasis group (p<0.01).
Diosmin is a member of the citrus flavonoid family and is a modified form of hesperidin [1-4]. It is a semisynthetic drug that is used as an oral phlebotropic drug in the treatment of venous disease, i.e., chronic venous insufficiency and hemorrhoidal disease. In some countries, Daflon 500 mg (which contains 90% diosmin+10% hesperidin) is used as a prescription medication [4]. Diosmin prolongs the vasoconstrictor effect of norepinephrine on the vein wall, increases the venous tone, and therefore reduces venous capacitance, distensibility, and stasis. Furthermore, it improves lymphatic drainage by increasing the frequency and intensity of lymphatic contractions and at the same time decreases the diameter of the lymphatic capillaries [1-4]. In addition to its use in vascular disease, diosmin has been found to be effective in mitigating hyperglycemia in diabetic rats [2]. It is also speculated that diosmin may have potential in the treatment of neurodegenerative diseases, such as Alzheimer's disease. Moreover, its anti-inflammatory and anti-apoptotic activity has been demonstrated in neuronal cells [5]. It has been mentioned that the anticancer, cardiovascular, and anti-inflammatory activities are the properties of citrus flavonoids [6]. Until now, however, clinical studies of the effects of diosmin have been inconclusive. Furthermore, only a limited number of articles have been published and few articles are available on the use of diosmin in renal diseases.
Kidney stones (nephrolithiasis) are a solid concretion or crystal aggregation formed in the kidneys from dietary minerals existing in the urine. A geographic belt has been described to explain the incidence of renal stones [7]. The Africo-Asian stone-forming belt stretches from Sudan, the Arab Republic of Egypt, Saudi Arabia, the United Arab Emirates, the Islamic Republic of Iran, Pakistan, India, Myanmar, Thailand, and Indonesia to the Philippines [7]. In this area of the world, the disease affects all age groups from less than 1 year old to more than 70 years old [7,8]. Because nephrolithiasis is a widespread disease, blocking the process of stone formation and finding new therapeutic options is an important area of study. The present study was therefore conducted to investigate the effects of diosmin in a rat model of calcium oxalate (CaOx) nephrolithiasis by use of stereological techniques. The quantitative data obtained showed the total volume of the deposited CaOx and structural changes to the kidney, including the total volume of the glomeruli, proximal and distal convoluted tubules (PCT and DCT), loop of Henle, collecting duct, interstitial tissue, and vessels. A rapid and simple stereological method was used to obtain the total quantities on one microscopic slide without the need for serial sectioning of the renal tissue. This method of quantitative microscopy provides efficient and reliable data.
Thirty female rats weighing 199±15 g were selected. The animals were treated according to the standard directive as recommended and approved by the research authorities of Shiraz University of Medical Sciences (license no. 1391-1). The rats were randomly divided into three groups of 10 animals each, which is acceptable for stereological studies [9,10]. The first group (control) did not receive any treatments. In groups two and three, nephrolithiasis was induced. The second group received distilled water by gavage, whereas the third group received diosmin (80 mg/kg/d) dissolved in a distilled water vehicle given by gavage for 21 days [10].
To induce an experimental model of CaOx nephrolithiasis, the rats were orally administered 2.5% (V/V) ethylene glycol+2.5% (W/V) ammonium chloride (2 mL/d) by gavage. In addition, the rats experienced a restriction on their intake of drinking water (20 mL/d) for 21 days [11].
After 21 days, the rats' kidneys were removed. The renal pelvis was dissected out and the kidney was weighed. The primary volume of the kidney, Vprimary, was measured by using the immersion method of Sherle [12]. A brief description is presented under Fig. 1. The kidney was fixed in neutral buffered formaldehyde for 2 days. Then the tissue shrinkage produced by fixation, processing, and staining was estimated. Estimation of the shrinkage and the length of the tubular structure of the kidney requires isotropic, uniform, random sections [13-15]. These sections were obtained by the orientator method. A brief description is presented under Fig. 2. The estimated shrinkage was also used for the estimation of the final kidney volume to avoid the consecutive sectioning that is required for the Cavalieri method. A description can be found under Fig. 2. After estimating the volume shrinkage [13], the final volume of the kidney (the reference space) was corrected by using the following formula:
Each sampled section was analyzed by using a video microscopy system that was made up of a microscope (E-200, Nikon, Tokyo, Japan) linked to a video camera, a computer, and a monitor to determine the parameters. Between 8 and 12 microscopic fields were selected in a systematic random manner. Afterwards, the stereological grids were superimposed on the images by means of the stereology software designed at Histomorphometry & Stereological Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
However, the volume density of each structure (its volume per unit volume of the kidney) was estimated by using the point-counting method [13-15]. A brief description is presented under Fig. 3. The volume density (Vv) of the renal cortex, the medulla, the glomeruli, the PCT, the DCT, the collecting ducts, the Henle's loop, the vessels, the connective tissue, and the CaOx deposition was obtained at the final magnification of ×1,800. The point-counting method used the following formula:
where P(structure) and P(total) represent the sum of the number of the points hitting the structure and all sampled fields of the kidney, respectively.
The mean and the standard deviation of the kidney volume and the volume of the cortex and medulla are presented in Table 1. No significant differences were observed between the groups.
The total volume of the glomeruli, PCT, DCT, Henle's loop, collecting duct, cortical and medullary degeneration, and fibrotic tissue in the different groups is shown in Table 2. As the table depicts, the volume of the interstitial tissues remained unchanged. However, after the induction of nephrolithiasis, the volume of the glomeruli, PCT, DCT, Henle's loop, collecting ducts, and vessels was reduced -57%, 34%, 35%, 58%, 54%, and 32%, respectively, in the rats (p<0.001). After treating the nephrolithiasis with diosmin, on average, -70% to 96% of the glomeruli, PCT, Henle's loop, collecting ducts, and vessels remained intact (p<0.01). Tissue degeneration was also seen after nephrolithiasis. Of course, the degeneration of the cortical tissue was 5-fold that of the medulla.
After treating the rats with nephrolithiasis with diosmin, less CaOx was deposited in the kidney. On average, the volume of CaOx deposition was -63% less in the rats with nephrolithiasis treated with diosmin (p<0.01).
In the nephrolithiasis+diosmin rats, degeneration in the renal cortical tissue and medulla was reduced -70% and 44% compared with that in the rats with untreated nephrolithiasis (p<0.01). The diameter of the capillaries and vessels in the cortex was reduced -7% and -12%, respectively, in the rats with nephrolithiasis (p<0.001). The diameter of the capillaries and vessels in the medulla remained unchanged. After treating the nephrolithiasis with diosmin, the reduction of the diameter of the cortical capillaries and vessels was recovered (Table 3).
The results of the present investigation revealed that diosmin massively reduces CaOx formation and also protects the renal tissue from damage in a rat model of nephrolithiasis. Formation of CaOx stones starts from the attachment of a crystal formed in the lumen of the renal tubules to the surface of the epithelial cells [16-23]. Most of the crystals formed in the lumen of tubules are discharged in the urine. In healthy individuals, the crystals that attach to the surface of the epithelial cells are digested by macrophages or cellular lysosomes. However, in patients with hyperoxaluria or crystal urine, the renal tubular cells are injured, thus allowing crystals to easily attach to them. Then, the crystals aggregate and grow, and finally a stone is formed [16-23]. It was mentioned by Wiessner et al. [22] that CaOx crystal deposition in the kidneys can promote inflammation and lead to fibrosis. Several studies have provided evidence that oxalate-induced toxicity is related to superoxide and lipid peroxidation. The oxalate-induced increase in free radical production appears to be related to cell death in a concentration-dependent manner [16-23]. Finding a therapy for preventing the CaOx deposition seems to be possible by controlling the above-mentioned mechanism, in particular controlling the tubular cell injury and the inflammation process. Ebisuno et al. [20] concluded that urinary glycosaminoglycans might play a critical role in preventing the adhesion of crystals by inhibiting the renal tubular cell injury. Moreover, Ito et al. [24] reported that the antioxidative effect of green tea decreases CaOx stone formation, osteopontin expression, and apoptosis and increases superoxide dismutase activity in the kidney tissues of rats. Also, Sarica et al. [25] reported a significant decrease in the mean level of apoptosis in the animals receiving vitamin E and allopurinol. In the present study, the protective role of diosmin on the renal structure was shown after nephrolithiasis. Diosmin has been used to prolong the vasoconstrictor effect of norepinephrine on the venous and lymphatic vessels. As a flavonoid, however, it also exhibits anti-inflammatory, free-radical scavenging, and antimutagenic properties [6]. In the present research, tissue degeneration was reduced after the diosmin treatment. This finding might be related to the multifactorial characteristic of diosmin. Capillary hyperpermeability is one of the primary processes of the inflammation. At the microcirculation level, diosmin reduces capillary hyperpermeability and increases capillary resistance by protecting the microcirculation from the damaging processes [1-4]. In addition, diosmin reduces the expression of endothelial adhesion molecules and inhibits the adhesion, migration, and activation of leukocytes at the capillary level [1-4]. It has been reported that these effects lead to a reduction in the release of inflammatory mediators, principally oxygen free radicals and some prostaglandins. In a recent article, Srinivasan and Pari [2] showed the ameliorative effect of diosmin against the oxidative stress in the kidney of diabetic rats. Reduction of the degenerative tissue as a result of using diosmin was another finding of the study. This finding might be explained by the reduction in tubular cell injury and inflammation after the treatment with diosmin. In addition, observation of more intact tubules after diosmin treatment in the present survey is an indicator of less tubular cell injury.
Another aspect of the present research was the reduction of the diameter of the capillaries and vessels in the cortex after nephrolithiasis and the recovery of this after diosmin treatment. As stated previously, diosmin prolongs the vasoconstrictor effect of norepinephrine on the vein. Note that this is a transient physiologic effect. The diameter reduction of the histological sections is a structural change that might be due to tissue fibrosis, because a significant reduction was seen in the cortex and not the medulla, which contains less fibrous tissue.
Figures and Tables
FIG. 1
Immersion method. (A) A laboratory glass was filled with normal saline, placed on the scale, and weighed. (B) The kidney was suspended by a thin thread in the laboratory glass. The kidney did not have to touch the bottom or sides of the jar. The new weight in grams minus the weight of the laboratory jar and normal saline divided by the specific gravity of the normal saline (-1.004) was considered as the primary volume of the kidney in cubic centimeters.
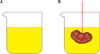
FIG. 2
Orientator method. (A) The kidney was placed on the circle such that each half of it was divided into 10 equal parts. A random number between 0 and 9 was selected. The kidney was sectioned into two pieces in the direction of the selected number (here 7). (B) The cut surface of one part of the kidney was then placed on the 0-0 direction of the second circle with 10 unequal divisions. The circle division was done according to the cosine of the angels. Then, another random number was selected and the second cut was done (here 1). The parts were sectioned into parallel slabs. (C) The cut surface of the other part of the kidney was placed vertically on the second circle. Again, a new number and direction (here 9) was selected and cut. (D) This part was also sectioned into parallels slabs in the direction of the isotropic uniform random cut at an interval of 0.5 mm. All the slabs (8-12 slabs) were collected. A circle was punched from a kidney slab by use of a trocar. The diameter of the circular piece and the area of the circle were estimated by using the usual formula for calculating the area of a circle. (E) The cut surface of the slabs and the circle were embedded in the paraffin block. (F) After staining with Heidenhain's Azan trichrome, the area of the circular piece was measured again. The microscopic fields were sampled in a systematic random design. The fields were sampled and analyzed at equal intervals along the X- and Y-axes by using a stage micrometer.
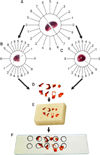
FIG. 3
The volume density estimation. A grid of points was superimposed on the live images of the renal sections. Stained with von Kossa. The volume density of the renal structures and the calcium oxalate deposition was obtained by using the point-counting method. The arrows indicate the calcium oxalate deposition in the cortex (A) and medulla (B). Scale bar is 40 µm.

TABLE 1
The mean (coefficients of variation) of the animals' weight, kidney weight, and volume in the control animals and the rats with nephrolithiasis with or without diosmin treatment

TABLE 2
The mean (coefficients of variation) of the volume of CaOx, Deg. cortex, Deg. medulla, GLOM, PCT & DCT, LH, CD, VES, and IT in the control animals and the rats with nephrolithiasis with or without diosmin treatment

CaOx, calcium oxalate; Deg. cortex, degenerative tissues in cortex; Deg. medulla, degenerative tissues in medulla; GLOM, glomeruli; PCT & DCT, proximal and distal convoluted tubules; LH, loop of Henle; CD, collecting duct; VES, vessels; IT, interstitial tissue.
a:p<0.001, nephrolithiasis vs. control. b:p<0.01, (nephrolithiasis+diosmin-treated) vs. nephrolithiasis.
ACKNOWLEDGMENTS
The present research was carried out at the Histomorphometry & Stereology Research Center of Shiraz University of Medical Sciences. The authors would like to thank Miss Elham Nadimi, Miss Zahra Keshavarz, and Mr. Mehrdad Azadi for their technical assistance. The Research Improvement Center of Shiraz University of Medical Sciences and Ms. Afsaneh Keivanshekouh are also appreciated for improving the use of English in the manuscript.
References
1. Tamamoto LC, Schmidt SJ, Lee SY. Sensory profile of a model energy drink with varying levels of functional ingredients-caffeine, ginseng, and taurine. J Food Sci. 2010. 75:S271–S278.
2. Srinivasan S, Pari L. Ameliorative effect of diosmin, a citrus flavonoid against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Chem Biol Interact. 2012. 195:43–51.
3. Jean T, Bodinier MC. Mediators involved in inflammation: effects of Daflon 500 mg on their release. Angiology. 1994. 45(6 Pt 2):554–559.
4. Labrid C. Pharmacologic properties of Daflon 500 mg. Angiology. 1994. 45(6 Pt 2):524–530.
5. Rezai-Zadeh K, Douglas Shytle R, Bai Y, Tian J, Hou H, Mori T, et al. Flavonoid-mediated presenilin-1 phosphorylation reduces Alzheimer's disease beta-amyloid production. J Cell Mol Med. 2009. 13:574–588.
6. Benavente-Garcia O, Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem. 2008. 56:6185–6205.
7. Lopez M, Hoppe B. History, epidemiology and regional diversities of urolithiasis. Pediatr Nephrol. 2010. 25:49–59.
8. Kohri K, Takada M, Katoh Y, Kataoka K, Iguchi M, Kurita T. Amino acids in urine and plasma of urolithiasis patients. Int Urol Nephrol. 1989. 21:9–16.
9. Hyde DM, Tyler NK, Plopper CG. Morphometry of the respiratory tract: avoiding the sampling, size, orientation, and reference traps. Toxicol Pathol. 2007. 35:41–48.
10. Bouskela E, Cyrino FZ, Lerond L. Leukocyte adhesion after oxidant challenge in the hamster cheek pouch microcirculation. J Vasc Res. 1999. 36:Suppl 1. 11–14.
11. Li CY, Deng YL, Sun BH. Taurine protected kidney from oxidative injury through mitochondrial-linked pathway in a rat model of nephrolithiasis. Urol Res. 2009. 37:211–220.
12. Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970. 26:57–60.
13. Nyengaard JR. Stereologic methods and their application in kidney research. J Am Soc Nephrol. 1999. 10:1100–1123.
14. Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, et al. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988. 96:857–881.
15. Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988. 96:379–394.
16. Corley RA, Meek ME, Carney EW. Mode of action: oxalate crystal-induced renal tubule degeneration and glycolic acid-induced dysmorphogenesis: renal and developmental effects of ethylene glycol. Crit Rev Toxicol. 2005. 35:691–702.
17. Saha S, Verma RJ. Bergenia ciliata extract prevents ethylene glycol induced histopathological changes in the kidney. Acta Pol Pharm. 2011. 68:711–715.
18. Khan SR. Calcium oxalate crystal interaction with renal tubular epithelium, mechanism of crystal adhesion and its impact on stone development. Urol Res. 1995. 23:71–79.
19. Tsujihata M. Mechanism of calcium oxalate renal stone formation and renal tubular cell injury. Int J Urol. 2008. 15:115–120.
20. Ebisuno S, Kohjimoto Y, Tamura M, Ohkawa T. Adhesion of calcium oxalate crystals to Madin-Darby canine kidney cells and some effects of glycosaminoglycans or cell injuries. Eur Urol. 1995. 28:68–73.
21. Scheid C, Koul H, Hill WA, Luber-Narod J, Kennington L, Honeyman T, et al. Oxalate toxicity in LLC-PK1 cells: role of free radicals. Kidney Int. 1996. 49:413–419.
22. Wiessner JH, Hasegawa AT, Hung LY, Mandel NS. Oxalate-induced exposure of phosphatidylserine on the surface of renal epithelial cells in culture. J Am Soc Nephrol. 1999. 10:Suppl 14. S441–S445.
23. Thamilselvan S, Byer KJ, Hackett RL, Khan SR. Free radical scavengers, catalase and superoxide dismutase provide protection from oxalate-associated injury to LLC-PK1 and MDCK cells. J Urol. 2000. 164:224–229.
24. Itoh Y, Yasui T, Okada A, Tozawa K, Hayashi Y, Kohri K. Preventive effects of green tea on renal stone formation and the role of oxidative stress in nephrolithiasis. J Urol. 2005. 173:271–275.
25. Sarica K, Yagci F, Bakir K, Erbagci A, Erturhan S, Ucak R. Renal tubular injury induced by hyperoxaluria: evaluation of apoptotic changes. Urol Res. 2001. 29:34–37.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download