Abstract
A 71-year-old man was referred for painless hematuria and a bladder tumor. Cystoscopy and computed tomography revealed a 3-cm oval nodular mass on the left lateral side of the bladder. The patient underwent a complete transurethral resection of the lesion and histology showed a proliferation of atypical spindle cells with inflammation consistent with a myofibroblastic tumor. After 4 and 7 months, follow-up cystoscopy demonstrated nodular mass lesions and transurethral resection of bladder tumor was done, which showed chronic cystitis and a recurred myofibroblastic tumor, respectively. Five months later, multiple lymph node, bone, and soft tissue metastases were found by positron emission tomography. The patient was treated first with palliative chemotherapy, including doxorubicin and cisplatin. After that, radiologic studies showed disease progression but the patient refused further treatment and died 6 months later.
Inflammatory myofibroblastic tumor (IMT) is a rare benign tumor with benign behavior. We reports an unusual case of recurrent IMT with malignant transformation with multiple metastasis.
A 71-year-old man was referred to Pusan National University Hospital with painless hematuria and a bladder tumor. He had no history of urinary tract infection, instrumentation, trauma, or surgery on the pelvis or abdomen.
A physical examination revealed slightly pale conjunctivas, but otherwise the results were unremarkable. Laboratory investigations showed a hemoglobin level of 9.1 g/dl, a leukocyte count of 14.65×109/l, a platelet count of 678×109/l, serum creatinine of 1.01 mg/dl, and blood urea nitrogen of 18.0 mg/dl. Other blood chemistry values were within normal limits. Routine urinalysis showed urine protein of 1+, urine red blood cells of 814.2/HPF, and urine white blood cells of 12.0/HPF. The results of urine cytology were negative for malignancy and acute inflammation.
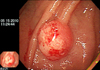
Contrast-enhanced computed tomography (CCT) showed a 3-cm oval nodular mass on the left lateral side of the urinary bladder (Fig. 1), and an approximately 3-cm sized polypoid mass with a wide stalk on the left lateral wall was visualized on cystoscopy (Fig. 2).
The patient underwent a complete transurethral resection (TUR) of the lesion. Histology revealed a proliferation of atypical spindle cells with inflammation consistent with a myofibroblastic tumor (Fig. 3). Immunohistochemistry demonstrated expression of endomysial antibody and cytokeratin. However, smooth muscle actin and anaplastic lymphoma kinase were not expressed.
After 4 months, a follow-up CT revealed a suspicious mass-like lesion on the left lateral wall of the bladder. A second TUR was performed. Pathology showed chronic inflammation with cystitis glandularis.
Three months later, a follow-up cystoscopy revealed a recurrent mass in the bladder neck and dome. A third TUR of bladder tumor was performed. Pathology revealed a proliferation of atypical spindle cells with inflammation, consistent with a myofibroblastic tumor and a recurred inflammatory myoblastic tumor (IMT). Immunohistochemistry was positive for cytokeratin and vimentin but negative for endomysial antibody (Fig. 4). However, no other mass lesion was found on the abdominal and pelvic CCT.
Five months later, the patient was transferred to Pusan National University Hospital for a bladder mass with suspicious multiple metastases and new-onset diabetes mellitus. An abdominopelvic CCT showed an approximately 3.2-cm extruding mass with central necrosis and peripheral enhancement in the anterior wall of the bladder. Multiple lymph node enhancements with a necrotic center in the mesenteric leaves were evident. A subcutaneous nodule was also noted in the right anterior abdominal wall (Fig. 5).
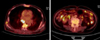
Positron emission tomography revealed multiple lymph node metastases in the left subclavian node, right axillary, mediastinum, diaphragm, and common iliac nodes. Multiple soft tissue metastases, bone metastases, carcinomatosis peritonei, and right pleural metastases were noted (Fig. 6). Endoscopy and colonoscopy findings showed multiple polypoid lesions in the duodenum and entire colon (Fig. 7). Pathology findings were compatible with metastatic IMT (Fig. 8). An ultrasound-guided extravesical mass biopsy was performed. The final diagnosis was IMT with malignant transformation. The patient was treated first with palliative chemotherapy, including doxorubicin and cisplatin. After that, he refused further treatment and died 6 months later.
IMT is a rare, benign spindle cell tumor with locally aggressive behavior [1]. IMT occurs in pulmonary and extrapulmonary sites, including the abdomen, retroperitoneum, head, neck, brain, and extremities. In the genitourinary tract, IMT has been reported in the kidney, urethra, prostate, ureter, and testis but is most frequently observed in the bladder [1].
On rare occasions, IMT may be locally aggressive, recurrent, or exhibit malignant transformation [2]. We described here an extremely unusual case of recurrent IMT with malignant transformation and multiple metastases originating from the bladder.
IMT is an uncommon benign neoplasm with locally aggressive behavior, but malignant changes are rare [1]. IMT of the bladder was initially reported by Roth in 1980 [3]. Most of the reported cases have occurred in young adults and there is a gender predilection for females (female to male ratio, 2:1) [4].
The clinical presentation varies depending on the anatomical location of the lesion [1]. In IMT of the bladder, painless hematuria with or without clots from exophytic and ulcerated lesions is the most common initial manifestation and may result in anemia [5]. In this case, the patient also had painless hematuria and anemia. Less often, patients present with dysuria, frequency problems, pelvic pain, or symptoms of a urinary tract obstruction. Infection of the lesion may be discernible as a mass lesion during a physical or radial examination [5]. Cystoscopic and radiologic examinations may reveal a single polypoid intraluminal mass or submucosal mass, ranging in size from 2 to 11 cm with or without an extension into perivesical fat [6].
IMT pathogenesis is contentious, and whether it is a post-inflammatory process or a true neoplasm remains unsettled [1]. Because IMT is characterized histologically by an inflammatory infiltrate, and various microbes have been isolated from lesions (such as mycobacteria, corynebacteria, the Ebstein-Barr virus, and human herpes virus), infection has long been suspected to play an important role in IMT pathogenesis [7]. Prior histories of surgery, trauma, and steroid use have also been associated with IMT [1]. In this case, there was no definite evidence of related infection, traumatic episode, or prior steroid use.
Anaplastic lymphoma kinase positivity is detected histopathologically in 36 to 60% of cases. An abnormality in anaplastic lymphoma kinase is most frequent in abdominal and pulmonary IMT in the first decade of life and is associated with a higher frequency of recurrence. IMT is also influenced by p53 mutations and "mouse double minute 2 homolog" (MDM2) expression [8]. IMT shows a wide spectrum of cellular atypia and biological behavior with p53 and MDM2 expression; however, these do seem to play a major role in IMT pathogenesis [8]. Cytokeratin is more likely to be expressed in bladder lesions than in lesions in some other site [9]. Thus, almost all IMT cases are diffusely positive for actin-associated proteins (e.g., smooth muscle actin, muscle-specific actin, calponin).
Radical resection is the treatment of choice for patients with IMT [10]. Recurrences are reported in 10 to 25% of genitourinary IMT cases [9]. In cases of a mesenteric or retroperitoneal origin of the tumor, large tumors (>8 cm) with multinodular ill-defined tumor morphology and incomplete tumor resection are known factors associated with a higher recurrence rate [1]. Little evidence exists regarding chemotherapy for IMT, and most data are from pediatric populations. Radiotherapy and even immunomodulation have not been reported to be consistently effective against aggressive IMT.
In this case, we planned to do radical cystectomy after the second recurrence. We monitored the patient every 3 months with cystoscopy and urine cytology. However, the pathology results of the third TUR were shown to be a second recurrence, and the patient was shown to have multiple metastases; thus, the patient underwent chemotherapy subsequently instead of surgery.
Most patients with IMT have an indolent course, and malignant change is very rare [1]. Lu et al. [10] reported a pelvic-abdominal IMT with malignant transformation in a 14-year-old boy. Although a radical tumor resection was performed, rapid tumor recurrence was noted after 20 days. Unlike that case, ours was a benign lesion at first, and malignant transformation with multi-organ metastasis was noted after several benign recurrences of IMT. Another case was reported showing malignant IMT in a 74-year-old man [9]. However, unlike our case, that patient had undergone prior irradiation owing to prostate cancer and this initiating step may have resulted in malignant IMT of the bladder and urethra.
Some rare cases have been reported indicating that urothelial carcinomas may be accompanied by reactive spindle cell proliferation in the stroma, which is referred to the pseudosarcomatous stromal reaction [9]. Montgomery et al. [9] described two cases of sarcomatoid carcinoma associated with high-grade invasive urothelial carcinoma accompanied by separated IMT fragments.
In summary, this report documents a rare case of bladder IMT with malignant transformation and multiple metastases in an older patient without previous cancer or radiotherapy. Although most IMTs are uncommon benign neoplasms, they can transform into malignant tumors. Close observation with regular follow-up should be emphasized.
Figures and Tables
FIG. 1
Contrast-enhanced computed tomography image showing a 3-cm mass on the left lateral side of the urinary bladder. Perivesical invasion was not seen.
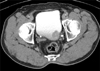
FIG. 2
An approximately 3-cm sized polypoid mass with a wide stalk on the left lateral wall was visualized by cystoscopy.

FIG. 3
The histopathological examination revealed a spindle cell tumor with abundant inflammatory cells in a background consisting of plasma cells, lymphocytes, and polymorphisms with no mitosis, compatible with inflammatory myoblastic tumor of the bladder.

FIG. 4
Pathology collected on the third transurethral resection of the bladder tumor revealed proliferation of atypical spindle cells with inflammation, consistent with a myofibroblastic tumor in recurring inflammatory myoblastic tumor. Immunohistochemistry was positive for cytokeratin and vimentin but negative for endomysial antibody.
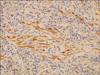
FIG. 5
Abdominopelvic contrast-enhanced computed tomography scan showing an approximately 3.2-cm extruding mass with central necrosis and peripheral enhancement in the anterior wall of the bladder. Multiple lymph node enhancement with a necrotic center in the mesenteric leaf was evident. A subcutaneous nodule was also noted on the right anterior abdominal wall.
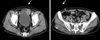
FIG. 6
Positron emission tomography scan showing multiple lymph node metastases in the left subclavian node and in the right axillary, mediastinum, diaphragm, and common iliac nodes. Multiple soft tissue, bone metastasis, carcinomatosis peritonei, and right pleural metastases were noted.
References
1. Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995. 19:859–872.
2. Dishop MK, Warner BW, Dehner LP, Kriss VM, Greenwood MF, Geil JD, et al. Successful treatment of inflammatory myofibroblastic tumor with malignant transformation by surgical resection and chemotherapy. J Pediatr Hematol Oncol. 2003. 25:153–158.
3. Roth JA. Reactive pseudosarcomatous response in urinary bladder. Urology. 1980. 16:635–637.
4. Young RH. Pseudoneoplastic lesions of the urinary bladder and urethra: a selective review with emphasis on recent information. Semin Diagn Pathol. 1997. 14:133–146.
5. Li HB, Xu YM, Yu JJ. Diagnostic puzzle of inflammatory pseudotumor of the urinary bladder: a case report with brief literature review. South Med J. 2010. 103:563–566.
6. Fujiwara T, Sugimura K, Imaoka I, Igawa M. Inflammatory pseudotumor of the bladder: MR findings. J Comput Assist Tomogr. 1999. 23:558–561.
7. Arber DA, Weiss LM, Chang KL. Detection of Epstein-Barr virus in inflammatory pseudotumor. Semin Diagn Pathol. 1998. 15:155–160.
8. Yamamoto H, Oda Y, Saito T, Sakamoto A, Miyajima K, Tamiya S, et al. p53 Mutation and MDM2 amplification in inflammatory myofibroblastic tumours. Histopathology. 2003. 42:431–439.
9. Montgomery EA, Shuster DD, Burkart AL, Esteban JM, Sgrignoli A, Elwood L, et al. Inflammatory myofibroblastic tumors of the urinary tract: a clinicopathologic study of 46 cases, including a malignant example inflammatory fibrosarcoma and a subset associated with high-grade urothelial carcinoma. Am J Surg Pathol. 2006. 30:1502–1512.
10. Lu CH, Huang HY, Chen HK, Chuang JH, Ng SH, Ko SF. Huge pelvi-abdominal malignant inflammatory myofibroblastic tumor with rapid recurrence in a 14-year-old boy. World J Gastroenterol. 2010. 16:2698–2701.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download