Abstract
Purpose
We assessed the efficacy and safety of insertion of a polytetrafluoroethylene membrane-covered self-expandable metallic stent (UVENTA stent) for palliation of malignant ureteral obstruction on the basis of our early results.
Materials and Methods
Eighteen patients underwent UVENTA stent insertion for extrinsic malignant ureteral obstructions of 20 ureters. The UVENTA stents were deployed retrogradely under cystoscopy and fluoroscopy. Candidates for the procedure had preexisting double-J stents that were nonfunctional or caused excessive bladder irritation. We recorded the success and patency rate in addition to any complications associated with the procedure.
Results
The mean length of obstruction was 10.6 cm (range, 2 to 20 cm). Two ureters were obstructed in the upper ureter, 9 in the lower ureter, and 9 in multiple levels of ureter. Simultaneous balloon dilation was performed in 12 ureters. UVENTA stents were successfully inserted in all patients. No obstruction of the UVENTA stents occurred during the mean follow-up period of 7.3 months (patency rate 100%), but de novo ureteral obstruction developed in 4 ureters. There were no instances of stone formation, hyperplastic reaction, encrustation, or migration. Abnormally elevated serum creatinine decreased to normal levels and hydronephrosis gradually resolved during the 4 weeks after UVENTA insertion. No significant complications developed except for transient and self-limiting hematuria and mild lower abdominal pain.
Extrinsic malignant ureteral obstruction may compromise ureteral patency and lead to renal failure [1,2]. Malignant unilateral or bilateral ureteral obstruction may be secondary to direct tumor invasion, extrinsic compression, or encasement by metastatic retroperitoneal or pelvic lymph nodes [3]. Malignant ureteral obstructions usually require immediate ureteral decompression in order to restore renal function [1,4]. The selection of cancer patients for diversion should take into account factors such as tumor stage, prognosis of the primary cancer, likelihood of additional antineoplastic therapy, and quality of life [5].
Contemporary management options include external drainage via percutaneous nephrostomy (PCN) and internal drainage via the insertion of double-J stents [6,7]. Regular double-J stents used to relieve ureteral obstructions that are secondary to extrinsic causes, such as malignancies, have high rates of failure [2,8]. PCN is commonly used as an alternative, either as a primary procedure or after the failure of transurethral procedures [9]. However, PCN is more invasive than double-J stent insertion and may also have a greater incidence of accidental tube dislodgement [10]. The invasiveness of the procedure and the high incidence of tube dislodgement may result in a reduction in patient quality of life. In addition, some patients are unwilling to accept a PCN tube because it requires an external collecting device. Both PCN and double-J stents must be periodically changed.
The limitations associated with conventional treatments for ureteral obstructions highlight the need for a novel treatment that can maintain ureteral patency while minimizing the deterioration of patient quality of life. Several types of metallic stents have been used in the palliative treatment of malignant ureteral obstructions, but implantation of these stents has yielded results that are not uniform and the stents have been associated with various complications. A novel polytetrafluoroethylene (PTFE) membrane-covered metal mesh stent prevents obstruction from tissue ingrowth and reduces stent migration as a result of its unique structure.
Here we report our initial experience with a PTFE membrane-covered self-expandable metallic stent (UVENTA stent) for the palliative care of malignant ureteral obstruction.
Between October 2010 and November 2011, 18 consecutive patients (5 men and 13 women; mean age, 57 years) underwent placement of UVENTA stents (Taewoong Medical, Seoul, Korea) for unilateral or bilateral malignant ureteral obstruction. The UVENTA stents were deployed retrogradely under cystoscopy and C-arm guidance.
The ureteral obstructions were caused by compression by a localized primary tumor in 1 ureter, remote metastatic disease or direct tumor infiltration in 6 ureters, and encasement by retroperitoneal lymphadenopathy in 13 ureters. Six patients had uterine cervical cancer, 7 had colorectal cancer, 4 had stomach cancer, and 1 had retroperitoneal sarcoma (Table 1). Diagnostic imaging of obstructions was performed by using transabdominal ultrasound, computerized tomography (CT), or intravenous urography (IVU). A single urologist (JWP) performed all of the stent placement procedures. All patients provided informed consent before undergoing the procedure. Candidates for UVENTA stent placement had pre-existing double-J stents that were either nonfunctional or caused excessive bladder irritation. Patients with ureteral stricture due to radiation therapy were not included in the study.
The UVENTA stent has two components: the stent and the delivery system. The stent has 3 layers. The outer and inner meshes are made of nickel plus titanium alloy and there is a PTFE membrane between them (Fig. 1C). The delivery system comprises two coaxially arranged shafts. The inner shaft is pierced to the tip by the central lumen to allow the passage of a 0.035-inch guidewire. The stent is mounted proximal to the tip of the inner shaft. The stent-loaded inner shaft is covered with a 9-Fr outer sheath. The stent is deployed by pulling the outer sheath back without moving the inner shaft. Stent position can be determined by using radiopaque markers (Fig. 1E) on the inner sheath. We used a UVENTA stent with a 7-mm nominal diameter that ranged in length from 6 to 12 cm.
All UVENTA stents were placed retrogradely under cystoscopy and C-arm guidance. Antibiotic prophylaxis was administered 12 hours before the procedure. Patients were placed in a lithotomy position after general anesthesia. Retrograde pyelography was performed to identify the obstructed ureteral segment shape, level, and length (Fig. 2A).
Stricture traversal was attempted by an appropriate combination of a 0.035-inch straight percutaneous transluminal angioplasty guidewire with hydrophilic coating or PTFE-coated guidewire and a 5.0 Fr/70 cm ureteral catheter (Open-End Flexi-Tip, Cook Urological Inc., Spencer, IN, USA). Gentle interventional maneuvers were necessary to prevent ureteral rupture and contrast extravasation, which might blur the fluoroscopic field and promote periureteral fibrosis. After successfully traversing the stricture, the guidewire was forwarded into the renal pelvis and exchanged for a rigid 0.035-inch Amplatz Super Stiff guidewire (Boston Scientific, Miami, FL, USA) to strengthen the ureteral course and secure the luminal passage during balloon dilation and stent placement (Figs. 2B, 2C). The stent was placed such that its upper and lower ends bypassed the stricture by 2 cm. When the obstruction site was in the distal ureter, the lower end of the stent was positioned intravesically, extending approximately 0.5 to 1 cm from the ureteral orifice (Fig. 1D). In long strictures requiring two or more UVENTA stents, the stents were placed sequentially, overlapping by 2 to 3 cm.
Balloon dilation was performed with a 6-mm balloon dilation catheter (UroMax Ultra, Boston Scientific, Galway, Ireland). Balloon length was chosen according to baseline lesion length. A single balloon was used in lesions ≤10 cm. Serial balloon inflation was performed in lesions >10 cm. Inflation pressure was increased until balloon waisting was abolished and ureteral continuity was established. Typical inflation pressure was 15 atm for 5 minutes. Repeated high-pressure balloon dilation (post-dilation pressure up to 20 atm) was necessary in cases of resistant stricture and suboptimal stent expansion.
After the intervention was completed, we planned follow-up visits with patients at 1, 3, 6, and 12 months after stent implantation and yearly thereafter. Urine culture, blood biochemistry tests, and IVU or CT were performed at the follow-up examinations to detect any recurrent strictures.
All patients received specific instructions to return to our hospital in case of ipsilateral flank pain, abdominal pain, fever, dysuria, urgency, frequency, hematuria, or vomiting. Additional UVENTA stent insertion was planned for any recurrent ureteral obstruction during follow-up.
Technical success was defined as successful traversal and stenting of the ureteral stricture. Clinical success was defined as an unobstructed stent with non-deteriorating renal function. Patency was defined as successful abolishment of stricture after stent implantation without additional intervention.
Two patients had bilateral ureteral obstructions, resulting in a total of 20 ureters that were managed by UVENTA implantation. Mean ureteral obstruction length was 10.6 cm (range, 2 to 20 cm). Of the 20 lesions, 9 were in the lower ureter.
The cystoscopic retrograde placement of the UVENTA stent was successful in all 18 patients. The first cystoscopic attempt failed in one patient because direct tumor invasion into the uretero-vesical junction made it impossible to find a ureteral orifice. The patient then underwent an anterograde double-J stent insertion, and we used the double-J stent as a guide for cystoscopic retrograde UVENTA insertion. We performed simultaneous balloon dilation in 12 ureters (60%) because there was insufficient expansion of the UVENTA stent (<50% of diameter) after deployment. The procedure time varied with the length and severity of obstruction, but ranged between 30 and 60 minutes. Abnormally elevated serum creatinine decreased to normal levels (Table 1) and hydronephrosis gradually resolved by 4 weeks after UVENTA insertion (Fig. 3).
Two patients experienced mild lower abdominal pain and discomfort for a few days after UVENTA deployment, probably as a result of the expanding force of the endoprostheses. Both patients recovered within 1 month without any treatment. Two patients reported irritative bladder symptoms that may have been due to the protrusion of the distal end of the UVENTA stent into the bladder. Irritative symptoms were controlled with anticholinergics. Three patients had transient gross hematuria that resolved spontaneously within 1 month.
Mean follow-up time was 7.3 months (range, 3 to 15 months). Follow-up imaging studies (CT or IVU) indicated no stent obstruction by hyperplastic reaction or tumor ingrowth and the UVENTA stents maintained their shape. There were no instances of stone formation, encrustation, or migration of the UVENTA stents. The overall patency rate was 100% during the follow-up period.
The first report of double-J stents as a treatment for ureteral obstruction was published in 1978. Subsequently, these stents have been widely used for patients with extrinsic ureteral obstructions due to malignancy [11,12]. However, the use of double-J stents in malignant ureteral obstructions has a high rate of failure [2,8].
Metallic stents have been applied in cardiac, gastroenterological, hepatobiliary, and vascular systems [13]. Several authors have proposed metal stents to ameliorate obstructive urinary tract pathology, including benign prostatic hyperplasia, urethral stenosis, ureteroileal anastomotic stricture, benign and malignant ureteral obstruction [4], and even kidney transplantation ureteral stenosis [14]. Self-expandable metallic stents have been used to treat malignant ureteral obstruction with acceptable results, but they can be obstructed by hyperplastic reactions or tumor ingrowth through the stent struts. In a description of their 10 years of experience with metal mesh stents, Liatsikos et al. [15] reported that the most common complications that jeopardized ureteral patency were hyperplastic reactions and tumor ingrowth. These complications developed in 45 of 119 ureters.
The externally coated ureteral metallic stents and thermo-expandable metallic ureteral stents that were introduced to limit the ingrowth of hyperplastic tissue through stent struts frequently migrate, with migration occurring in 17.5 to 81.2% of these stents [16,17]. This stent migration may be attributed to the lack of an appropriate anchor to the ureteral wall for the prostheses and propulsion by anterograde peristalsis.
The unique structure of the UVENTA stents used in the present study prevents not only tissue ingrowth but also stent migration. The inner mesh and PTFE membrane prevent tissue ingrowth into the stent and help to maintain patency of the stent. The outer mesh induces ingrowth of tissue that acts as an anchor between the stent and the ureteral wall, thereby preventing migration. In a study comparing PTFE-covered metallic stents with uncovered metallic stents in canine ureters, Chung et al. [18] observed tissue proliferation on the outside of the covered stents but not within the lumen. This result showed that the PTFE membrane was effective at preventing tissue ingrowth. We found no evidence of migration or obstruction of the stents in any of the follow-up imaging studies.
An important complication associated with long-standing double-J stents is encrustation and stone formation with subsequent urinary tract infection and obstruction. Stent encrustation rates were reported to be 12 to 27% in series of studies with Memokath 051 thermoexpandable metallic stents [19,20]. The encrustation rates of covered metal mesh stents are not yet well known. Because metal stents are usually implanted in situ rather than being regularly changed like double-J stents, encrustation is a serious concern. In the present study, although we did not search for stone formation or encrustation by use of endoscopy, there was no stent obstruction from stones or visible encrustation in radiologic studies.
Some of the patients in this study experienced minor postoperative complications, such as gross hematuria, abdominal pain, or irritative urinary symptoms. However, there were no serious complications of grade III or higher by Clavien classification [21].
Variable patency rates (51.2 to 100%) are reported for various types of metallic ureteral stents used in the treatment of ureteral obstruction irrespective of cause [15,17,22,23]. In our study, the UVENTA stents were not obstructed during follow-up, so that the overall patency rate was 100%. However, de novo ureteral obstruction by tumor progression occurred in 4 ureters: 3 adjacent to the lower end, and 1 adjacent to the upper end of the implanted UVENTA stent. Additional UVENTA stent insertion relieved the obstructions in 2 patients. The other 2 patients were in the terminal stage of cancer, and no additional procedure was performed.
Some points of technique deserve special mention. In previous studies, several investigators performed metallic stent insertion by use of a percutaneous anterograde approach [15,22]. However, a percutaneous approach requires the assistance of an interventional radiologist and an additional invasive PCN procedure. Many urologists are already familiar with the cystoscopic retrograde double-J stent insertion, and the procedure for insertion of UVENTA stents is virtually the same. We performed all procedures by using a retrograde approach and easily implanted the stents in all but one patient. If the obstruction is in the lower ureter and uretero-vesical junction and may be due to invading cancer, it is helpful to use cystoscopy to identify an ipsilateral ureteral orifice before the procedure. When such an orifice cannot be identified, preoperative PCN and anterograde double-J stent insertion is necessary for successful UVENTA stent implantation. When the obstruction site is in the upper or midureter, it is better to conduct balloon dilatation after UVENTA insertion because the proximal and the distal ends of the stricture site cannot be accurately identified after balloon dilation is complete. When the obstruction is in the distal ureter, we advise positioning the lower end of the stent intravesically, extending approximately 0.5 to 1 cm from the ureteral orifice. This will reduce the risk of possible uretero-vesical junction obstruction by later tumor invasion.
The recently developed UVENTA stent prevents tumor ingrowth through the mesh and is resistant to stent-related complications. Therefore, it obviates the need for regular stent changes and thus offers significant benefits for patients with limited life expectancy.
There are several limitations of this study. The duration of follow-up was relatively short. Although we did not find any serious stent-related complications in the short term, a longer follow-up period is required to confirm the safety and efficacy of UVENTA stent insertion. Additionally, we did not evaluate stent-related symptoms and quality of life changes by use of validated questionnaires. It is essential to determine whether there are any benefits of UVENTA stents over double-J stents by conducting a comparative study. We are planning a prospective comparative study of this type to better evaluate the potential of UVENTA stents for the treatment of ureteral obstruction.
Figures and Tables
FIG. 1
Structure of the polytetrafluoroethylene membrane-covered self-expandable metallic (UVENTA) stents. (A) Both ends are fully covered with polytetrafluoroethylene membrane. (B) The flexibility of the UVENTA stent is shown. (C) The 2 metallic meshes (outer & inner) with the polytetrafluoroethylene membrane between them. (D) Cystoscopic view of the UVENTA stent after deployment in the trans-uretero-vesical junction. (E) Components of the inner sheath.
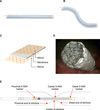
FIG. 2
Steps for insertion of polytetrafluoroethylene membrane-covered self-expandable metallic (UVENTA) stents. (A) Using retrograde pyelography, the length, shape, and level of stricture in the left upper ureter are identified. (B) The obstructed ureter is dilated by using a balloon dilator before UVENTA stent insertion. (C) The UVENTA stent is deployed. Note that 2 stents are sequentially inserted with overlapping ends in order to cover a long stricture.
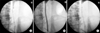
FIG. 3
Intravenous urography before and 4 weeks after polytetrafluoroethylene membrane-covered self-expandable metallic (UVENTA) stent insertion to treat a right lower ureter obstruction due to local recurrence of right colon cancer. (A) Before UVENTA insertion. Note hydronephrosis in right kidney despite the double-J stent. (B) Four weeks after UVENTA insertion. The 8 cm-long UVENTA stent is in the right lower ureter. Hydronephrosis is no longer observed.
TABLE 1
Patient characteristics and the results of UVENTA stent insertion

L, left; R, right; B, both; CR, colorectal; S, stomach; C, cervix; LM, leiomyosarcoma; LU, lower ureter; MU, multiple levels of ureter; UU, upper ureter; Pre, preoperative value; Post, postoperative value (4 weeks after stent insertion); HM, hematuria; IR, irritation; AD, abdominal discomfort.
a:For de novo obstruction beyond the previous UVENTA stent.
References
1. Yachia D. Recent advances in ureteral stents. Curr Opin Urol. 2008. 18:241–246.
2. Chung SY, Stein RJ, Landsittel D, Davies BJ, Cuellar DC, Hrebinko RL, et al. 15-year experience with the management of extrinsic ureteral obstruction with indwelling ureteral stents. J Urol. 2004. 172:592–595.
3. Russo P. Urologic emergencies in the cancer patient. Semin Oncol. 2000. 27:284–298.
4. Liatsikos EN, Karnabatidis D, Kagadis GC, Katsakiori PF, Stolzenburg JU, Nikiforidis GC, et al. Metal stents in the urinary tract. EAU-EBU Update Series. 2007. 5:77–88.
5. Emmert C, Rassler J, Kohler U. Survival and quality of life after percutaneous nephrostomy for malignant ureteric obstruction in patients with terminal cervical cancer. Arch Gynecol Obstet. 1997. 259:147–151.
6. Aravantinos E, Anagnostou T, Karatzas AD, Papakonstantinou W, Samarinas M, Melekos MD. Percutaneous nephrostomy in patients with tumors of advanced stage: treatment dilemmas and impact on clinical course and quality of life. J Endourol. 2007. 21:1297–1302.
7. Russo P. Ureteral obstruction and stents: still a difficult problem for patients and urologists alike. J Urol. 2005. 174:2088.
8. Ganatra AM, Loughlin KR. The management of malignant ureteral obstruction treated with ureteral stents. J Urol. 2005. 174:2125–2128.
9. Watkinson AF, A'Hern RP, Jones A, King DM, Moskovic EC. The role of percutaneous nephrostomy in malignant urinary tract obstruction. Clin Radiol. 1993. 47:32–35.
10. Lau MW, Temperley DE, Mehta S, Johnson RJ, Barnard RJ, Clarke NW. Urinary tract obstruction and nephrostomy drainage in pelvic malignant disease. Br J Urol. 1995. 76:565–569.
11. Finney RP. Experience with new double J ureteral catheter stent. J Urol. 1978. 120:678–681.
12. Hepperlen TW, Mardis HK, Kammandel H. The pigtail ureteral stent in the cancer patient. J Urol. 1979. 121:17–18.
13. Gillams A, Dick R, Dooley JS, Wallsten H, el-Din A. Self-expandable stainless steel braided endoprosthesis for biliary strictures. Radiology. 1990. 174:137–140.
14. Burgos FJ, Pascual J, Marcen R, Garcia-Navas R, Garcia IG, Alarcon C, et al. Self-expanding metallic ureteral stents for treatment of ureteral stenosis after kidney transplantation. Transplant Proc. 2005. 37:3828–3829.
15. Liatsikos EN, Karnabatidis D, Katsanos K, Kallidonis P, Katsakiori P, Kagadis GC, et al. Ureteral metal stents: 10-year experience with malignant ureteral obstruction treatment. J Urol. 2009. 182:2613–2617.
16. Barbalias GA, Liatsikos EN, Kalogeropoulou C, Karnabatidis D, Zabakis P, Athanasopoulos A, et al. Externally coated ureteral metallic stents: an unfavorable clinical experience. Eur Urol. 2002. 42:276–280.
17. Agrawal S, Brown CT, Bellamy EA, Kulkarni R. The thermo-expandable metallic ureteric stent: an 11-year follow-up. BJU Int. 2009. 103:372–376.
18. Chung HH, Lee SH, Cho SB, Park HS, Kim YS, Kang BC, et al. Comparison of a new polytetrafluoroethylene-covered metallic stent to a noncovered stent in canine ureters. Cardiovasc Intervent Radiol. 2008. 31:619–628.
19. Arya M, Mostafid H, Patel HR, Kellett MJ, Philp T. The self-expanding metallic ureteric stent in the long-term management of benign ureteric strictures. BJU Int. 2001. 88:339–342.
20. Klarskov P, Nordling J, Nielsen JB. Experience with Memokath 051 ureteral stent. Scand J Urol Nephrol. 2005. 39:169–172.
21. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004. 240:205–213.
22. Tekin MI, Aytekin C, Aygun C, PeSkircioglu L, Boyvat F, Ozkardes H. Covered metallic ureteral stent in the management of malignant ureteral obstruction: preliminary results. Urology. 2001. 58:919–923.
23. Trueba Arguinarena FJ, Fernandez del Busto E. Self-expanding polytetrafluoroethylene covered nitinol stents for the treatment of ureteral stenosis: preliminary report. J Urol. 2004. 172:620–623.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download