Abstract
Purpose
Testicular microlithiasis (TM) is an uncommon pathologic condition that is commonly diagnosed by scrotal ultrasonography. Indirect evidence suggests that this syndrome may be associated with an increased risk of testicular malignancy and infertility.
Materials and Methods
A total of 1,439 patients undergoing scrotal ultrasound during a 6-year, 5-month period (January 2003 to May 2009) were retrospectively reviewed. Any possible association of TM with pathologic findings was assessed. Among patients with TM, further grading of TM with testicular cancer and semen analysis of the infertile group with TM were also performed.
Results
TM was diagnosed in 87 patients (6.0%) out of a total of 1,439. Of all established pathologic entities, only testicular malignancy and infertility were meaningfully associated with TM. There was no significant difference in the prevalence of testicular cancer between each grade. Seminal profiles (sperm count, motility, morphology, and white blood cell count) were not found to be statistically different between infertile men with and without TM.
Conclusions
The prevalence of TM in symptomatic men was found to be 6.0% with significant co-occurrence of TM, testicular cancer, and infertility. Further grading of TM does not seem to be essential with regard to the detection of patients with testicular cancer and TM. TM showed no significant effect on the seminal profiles of infertile men.
Testicular microlithiasis (TM) is an uncommon pathologic condition that is commonly diagnosed by scrotal ultrasonography [1,2]. The characteristic ultrasound (US) appearance of TM involves multiple bright foci, 1 to 2 mm in diameter, that are limited to the testicles. There is little or no acoustic shadowing, and microliths are either randomly distributed throughout the testicle or limited to only part of the testicle. TM has been seen in patients with testicular malignancy or various nonmalignant entities such as cryptorchidism, varicoceles, testicular torsion, Klinefelter's syndrome, pulmonary alveolar microlithiasis, neurofibromatosis, AIDS, intratubular germ cell neoplasia, and most frequently, infertility [3,4]. The clinical importance of TM arises from its possible association with testicular cancer and infertility [3,5-8]. Priebe and Garret first reported the phenomenon in 1970, after seeing bilateral, diffuse testicular calcification on a pelvic X-ray of a 4-year-old boy [9]. Since then, other authors have tried to link pathological findings of testicular calcification in testicular tumors and infertility to the US diagnosis of TM. Despite these reports, however, the relationship between TM and testicular cancer and infertility has been largely anecdotal and without predictive value. The lack of current proven follow-up strategies for patients with TM basically stems from ignorance of the exact prevalence of symptomatic and asymptomatic populations and ambiguity in the cause-effect relationship of TM with other potentially associated conditions.
The objectives of the present study were as follows: (1) to report the prevalence of TM in a symptomatic population from the Republic of Korea who were undergoing testicular ultrasonography; (2) to identify associated pathologic entities, especially testicular cancer and infertility; (3) to evaluate the role of further TM grading in the prevalence of associated testicular cancer; and (4) to study the seminal profile of TM patients.
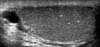
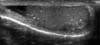
All patients (n=1,439) undergoing scrotal US during a 6-year, 5-month period (January 2003 to May 2009) were included. Scrotal ultrasonography was done in longitudinal and transverse sections by using a scanning device with a high-frequency linear array transducer and a center frequency of 14 MHz (SIEMENS ACUSON Sequoia 512 system, Germany). In patients without infertility, the indications for US were scrotal signs and symptoms such as palpable mass, pain, and swelling. All pathologic entities of patients who had undergone US were recorded. To identify possible associations with TM, analysis was performed by retrospective review of the medical records and US images. We defined TM as multiple (more than 2) calcifications smaller than 2 mm (no acoustic shadowing) inside the testicular parenchyma on US [3,4,10]. The presence of TM, the number of lesions, the involvement of both testicles in relation to symptoms, and the coexistence of other lesions were studied. Patients with TM were divided as follows: 3 or 4 microliths=limited (Fig. 1); 5-10 microliths=grade 1 (Fig. 2); 11-20 microliths=grade 2 (Fig. 3); and >20 microliths=grade 3 (Fig. 4) [11,12]. Solitary and two microcalculi or multiple coarse calcifications were not included in the analysis. We also recorded laterality of TM in addition to grade. In cases of testicular cancer accompanying TM, specific tumor types were pathologically confirmed.
In the infertile group, all were unable to conceive over the course of 1 year. Semen analysis was performed according to the World Health Organization criteria of 1999. This analysis included sperm count, motility, morphology, and white blood cell (WBC) count.
All statistical analyses were performed with SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were compared by chi-square test and a student's t-test was used for comparison of seminal profile. p-values of <0.05 were considered to indicate a statistically significant difference.
Of the 1,439 patients (mean age, 19.1±20.2 years; range, 0-87 years) who underwent scrotal US during the study period, various pathologic findings were found such as testicular cancer (n=57, 4.0%), hydrocele (n=547, 38.0%), cryptorchidism (n=310, 21.5%), epididymitis (n=199, 13.8%), varicocele (n=143, 9.9%), testicular atrophy (n=46, 3.2%) including epididymal cysts or dilation, testicular fibrosis, and normal manifestation (Table 1).
Testicular microlithiasis was diagnosed in 87 of the 1,439 patients (6.0%). The mean age of these patients was 26.2±17.9 years (range, 0-74 years). Among them, testicular cancer (n=15, 17.2%), hydrocele (n=31, 35.6%), varicocele (n=14, 16.1%), cryptorchidism (n=7, 8.0%), epididymal cysts (n=6, 6.9%), epididymitis (n=6, 6.9%), fibrosis, atrophy, inguinal hernia, and epididymal dilation were found. Of the 87 patients with TM, 18 (20.7%) were found to have limited TM, 16 were grade 1 (18.4%), 26 were grade 2 (29.9%), 27 were grade 3 (31.0%), and unilateral and bilateral TMs were detected in 28 (32.1%) and 59 (67.8%) patients, respectively. All detected TMs were ipsilateral to the associated abnormalities.
In 15 patients with testicular cancer and TM, the histopathologic diagnosis was classic seminoma in 6 patients, 6 mixed germ cell tumors, 2 teratomas, and 1 yolk sac tumor. All 15 patients with testicular cancer and TM had classic TM, which meant more than five microliths per transducer field. Applying this to the grading system, it was found that there was no limited TM in testicular cancer with TM. Of the 15 patients with testicular cancer and TM, grades 1, 2, and 3 consisted of 3 (20%), 7 (46.7%), and 5 (33.3%) patients, respectively (Fig. 5).
There was no significant difference in the prevalence of testicular cancer between grades (p=0.72). Among the US examinations performed in 60 patients, 10 cases were accompanied by TM (16.6%). Of all established pathologic entities, only testicular cancer was meaningfully associated with TM (Table 2). The prevalence of TM in testicular cancer was 26.3% (15/57) compared with 5.2% (72/1,382) in patients without testicular cancer (p<0.001).
In 60 cases of infertility (mean age, 33.7±4.6 years; range, 27-45 cases), 33 had varicocele (55%) and the rest had testicular atrophy, epididymal cysts, testicular fibrosis, and other afflictions.
All patients showed abnormal semen quality in semen analysis according to World Health Organization criteria. Among them, 10 cases were accompanied by TM, a prevalence rate of 16.7% (10/60) compared to 5.6% (77/1,439) in others (p=0.002) (Table 2). Of the 10, 5 were found to be limited TM, 1 was grade 1, 2 were grade 2, and 2 were grade 3 TM. There was no significant difference in the presence of other pathologies in those with or without TM (p=0.68). Furthermore, seminal profiles (sperm count, motility, morphology, and WBC count) were not statistically different between infertile men with and without TM (Table 3).
Testicular microlithiasis is a rare, asymptomatic finding suggested to be associated with various benign and malignant urological pathologies and genetic anomalies that are usually found incidentally during unrelated US examinations. Doherty et al reported the first use of real-time US to diagnose TM [2]. In the current study, the prevalence of TM was found to be 6.0% (87/1,439). The reported prevalence of TM has varied widely and has been generally presented as 0.5% to 9%. In their retrospective review of 1710 scrotal US scans, Höbarth et al documented 11 cases of TM (0.6%) in a population undergoing examination for varicocele, hypogonadism, and epididymal cysts [13]. In their retrospective review of 1,100 US scans performed for infertility, pain, or masses, Ganem et al found TM in 2% [14]. Middleton et al reviewed US data from 1,079 patients at their institution and found a prevalence of 0.68% [15]. This wide range partially comes from the recent introduction of high-frequency probes and the sensitization of radiologists and urologists to the problem [2]. But much more importantly, the vast majority of studies have investigated patients examined for testicular pathology. Virtually no true screening test for TM in an asymptomatic population has ever been performed except in two published reports. Peterson et al performed screening US scans on 1,504 healthy nonsymptomatic males and reported a prevalence of 5.6% [16]. More recently, Serter et al found the prevalence of TM in an asymptomatic population to be 2.4% after screening 2,179 US scans [17]. Despite these findings, the true incidence in the general population remains unclear.
Although the true incidence of TM is not yet known, TM has become a concern for the practicing urologist because of its possible correlation with testicular cancer. The coexistence of many benign and malignant pathologies with TM has been reported, and some authors have recognized that TM may be associated with a variety of nonneoplastic conditions, such as cryptorchidism, chromosomal anomaly, and atrophy. However, the most commonly held views in the literature are those that support a correlation between TM and testicular cancer [5,15,18].
We retrospectively reviewed all US cases and evaluated whether there was any possible significant association between TM and pathologic entities. Of all detected testicular pathologies, only testicular cancer showed a significant difference in prevalence. Previous ultrasonography studies showed a prevalence of TM ranging from 16.9% to 48.3%. In our study, we found 15 cases with TM out of 57 (26.3%) testicular cancers versus 72 cases with TM out of 1,382 (5.2%) patients without testicular cancer (p<0.001). Historically, frequently reported testicular cancer subtypes have been seminoma, teratoma, and mixed germ cell tumors in decreasing order. However, we found six patients with seminoma and another six with mixed germ cell tumors.
The correlation between TM and testicular tumors has been documented extensively in the literature [11,13,16]. The association between TM and malignancy was first highlighted in 1982 by Ikinger et al, who examined testicular specimens from 92 postoperative patients (43 with malignant and 49 with nonmalignant disease) [19]. Microcalcifications were found in 32 (74%) tumor specimens but only 8 (16%) benign specimens. Höbarth et al found tumors coexisting with TM in 44% of the 42 cases of TM [13]. Backus et al, in a cross-sectional retrospective study, reviewed 42 cases of TM and their association with identified intratesticular abnormalities. They noted that 40% of patients with TM on US had associated tumors [11]. In a larger series, Otite et al reviewed the records of 3,026 patients who had undergone scrotal US. Thirty percent of their patients with TM were diagnosed with testicular tumors [18]. On the other hand, Song et al reviewed 1,088 patients who underwent ultrasonography [20]. In that study, TM was found in 6% and 5.8% of the testicular cancer patients and the noncancer controls, respectively, in the children's group (p=0.152). In the adult group, 11.6% and 3.3% of the patients in the respective groups were found to have TM (p=0.001) [20]. To overcome the limitations of the majority of published reports, which have been retrospective in nature, several authors have performed prospective follow-up studies of patients with TM. Although many published reports found subsequent testicular cancer development in men who had been previously diagnosed with TM [4,18,21], the relationship between TM and testicular cancer remains unresolved due to several drawbacks of those studies. The majority of the prospective studies that have reported subsequent tumor occurrences were performed in a relatively small group (mostly less than 40) and involved short follow-up periods (mostly less than 4 years). More importantly, by carefully analyzing the reports that found subsequent tumor occurrence in patients with TM, most cases had other predisposing factors for the development of testicular tumors, such as cryptorchidism, testicular atrophy, or previous contralateral testicular tumors. Finally, several other follow-up studies have not shown new tumor cases [4,11,14,16].
Another important aim of our study was to evaluate the impact of grading on the prevalence of TM. Because there has been no accurate prevalence and a lack of agreed upon clinical importance of TM, further classifying was suggested to help to understand the nature of TM. Generally, TM has been accepted as classic TM when at least one image shows five or more microliths [10]. Patients who have at least one microliths and do not meet the criteria for classic TM have been considered to have limited TM [12]. Further classification of TM as done in our study was also suggested by Backus et al [11]. Presently, the number of microliths required to obtain a diagnosis of TM has been agreed upon at five and above for each sonographic plane [11,13]. However, in cases with smaller numbers of microliths that were associated with malignancy [22], we documented all microliths except 1 or 2 that could be an incidental finding without pathological significance. As we investigated the further grading of TM according to the number of microliths per US image, we found no difference in the prevalence between grades 1, 2, and 3 in accordance with the reports by Sanli et al [10]. In that study, they reviewed 4,310 cases with classic TM and further graded TM as in the present study; however, there was no significant difference between grades. Bennett et al compared the rates of testicular cancer in patients with 5 to 10 microliths per image (grade 1) with those having more than 10 microliths per image (grade 2 and 3) and reported no significant difference between the two groups [23]. Therefore, we concluded that further grading of TM is not essential in patients with TM. But this should be carefully interpreted because the relatively small number of cases of testicular cancer with TM may limit the power of this result. Because there were only five bilateral TM cases in 15 tumor cases with TM, we couldn't determine the clinical significance of laterality with respect to TM. Several other studies have shown that bilateral TM is associated with the pre-invasive stage of germ cell testicular cancer more so than unilateral [5,24].
The relationship between TM and infertility is not well understood. Testicular microlithiasis, which is frequently seen with testicular cancer, may be associated with infertility [5-8]. Theoretically, decreased fertility could be expected because 30% to 60% of seminiferous tubules can be obstructed by intratubular concretions, which is considered to be a pathogenesis of TM. Infertile patients with TM may have significant reductions in sperm migrations and motility compared with those with minimal microcalcification [7]. However, although some authors have reported abnormal semen parameters in infertile men with TM [1,8], others have found no significant difference among infertile men with or without TM [13,25]. In the present study, we found a prevalence rate of TM of 16.6% (10/60) in the infertile group, which fits into the currently reported prevalence (0.8 to 20%) and confirms a significant co-occurrence (p=0.002). There were no significant differences in sperm count, motility, or morphology in terms of sperm function between infertile men with or without TM. Our results suggest that, although there is a significant association between TM and infertility, it seems unwise to expect an adverse effect of TM on seminal profile. However, this could be confounded by other accompanying pathologies like varicoceles. We also assume that the relatively low grade of TM in the infertile group might have affected the seminal profile comparison. Thomas et al reported that cases with minor degrees of calcification generally had better sperm count and sperm migration tests than did those with marked calcification. There may therefore be a relationship between the degree of calcification and poor sperm function [26]. On the other hand, in the study by Sakamoto et al, which showed a similar seminal profile comparison result as in the present study, the authors mentioned that the degree of subfertility in patients with TM was variable and may reflect underlying testicular dysgenesis or concomitant intrascrotal abnormality [25].
Apart from the adverse effect on seminal profile, TM itself might be a risk factor for testicular cancer in infertile men who are already in a high-risk group [5,6]. In a study of 263 subfertile men by de Gouveia Brazao et al, the authors demonstrated that carcinoma in situ (CIS) was present in 20% of infertile men with bilateral TM. This was compared to 1% in the overall infertile population [5]. Patients with TM and infertility might have two risk factors for testicular cancer. Skakkebaek et al interpreted this increased risk for developing malignancy as the so-called testicular dysgenesis syndrome [27]. Those authors suggested that in men with TM, or other criteria for testicular dysgenesis such as testicular maldescent, atrophy, low sperm count, or inhomogeneous US appearance, the risk for CIS should be examined [28]. Testicular microlithiasis, infertility, and testicular cancer all therefore seem to be interlinked. Under these circumstances, it seems convincing that particular attention should be paid to TM patients who already have significant risk factors for the development of testicular cancer, such as infertility, cryptorchidism, or infertility, especially in diffuse bilateral cases [3,29].
This study had the following limitations. First, it was a retrospective review and was based on a preselected population undergoing US for myriad conditions that have the potential for increased incidence of germ cell tumors, which limits the power of the results. Second, we presented the prevalence of TM on the basis of a symptomatic population. Together with the retrospective nature of the present study, this makes it difficult to determine the true incidence and natural course of TM. To the best of our knowledge, only two prospective studies are available to determine the incidence and natural history of TM in a healthy asymptomatic population [14,15].
The prevalence of TM in the symptomatic population in our study was found to be 6.0%. Our study indicates the significant co-occurrence of TM, testicular cancer, and infertility.
Even if a true cause-effect relationship between TM and testicular cancer exists, further grading of TM does not seem to be essential with regard to the detection of patients with testicular cancer and TM. Furthermore, TM showed no significant impact on seminal profiles in infertile men in the present study; however, it should be carefully interpreted with respect to other accompanying pathologies.
Despite a significant pool of knowledge, the natural history and clinical significance of TM are not well understood. To reach a definitive conclusion about TM, further larger prospective and collaborative studies are required.
Figures and Tables
References
1. Sasagawa I, Nakada T, Kazama T, Satomi S, Katayama T, Matuda S. Testicular microlithiasis in male infertility. Urol Int. 1988. 43:368–369.
2. Doherty FJ, Mullins TL, Sant GR, Drinkwater MA, Ucci AA Jr. Testicular microlithiasis. A unique sonographic appearance. J Ultrasound Med. 1987. 6:389–392.
3. Rashid HH, Cos LR, Weinberg E, Messing EM. Testicular microlithiasis: a review and its association with testicular cancer. Urol Oncol. 2004. 22:285–289.
4. Kocaoğlu M, Bozlar U, Bulakbaşi N, Sağlam M, Uçöz T, Somuncu I. Testicular microlithiasis in pediatric age group: ultrasonography findings and literature review. Diagn Interv Radiol. 2005. 11:60–65.
5. de Gouveia Brazao CA, Pierik FH, Oosterhuis JW, Dohle GR, Looijenga LH, Weber RF. Bilateral testicular microlithiasis predicts the presence of the precursor of testicular germ cell tumors in subfertile men. J Urol. 2004. 171:158–160.
6. von Eckardstein S, Tsakmakidis G, Kamischke A, Rolf C, Nieschlag E. Sonographic testicular microlithiasis as an indicator of premalignant conditions in normal and infertile men. J Androl. 2001. 22:818–824.
7. Jacobsen R, Bostofte E, Engholm G, Hansen J, Olsen JH, Skakkebaek NE, et al. Risk of testicular cancer in men with abnormal semen characteristics: cohort study. BMJ. 2000. 321:789–792.
8. Aizenstein RI, DiDomenico D, Wilbur AC, O'Neil HK. Testicular microlithiasis: association with male infertility. J Clin Ultrasound. 1998. 26:195–198.
9. Priebe CJ Jr, Garret R. Testicular calcification in a 4-year-old boy. Pediatrics. 1970. 46:785–788.
10. Sanli O, Kadioglu A, Atar M, Acar O, Nane I, Kadioglu A. Grading of classical testicular microlithiasis has no effect on the prevalence of associated testicular tumors. Urol Int. 2008. 80:310–316.
11. Backus ML, Mack LA, Middleton WD, King BF, Winter TC 3rd, True LD. Testicular microlithiasis: imaging appearances and pathologic correlation. Radiology. 1994. 192:781–785.
12. Bushby LH, Miller FN, Rosairo S, Clarke JL, Sidhu PS. Scrotal calcification: ultrasound appearances, distribution and aetiology. Br J Radiol. 2002. 75:283–288.
13. Höbarth K, Susani M, Szabo N, Kratzik C. Incidence of testicular microlithiasis. Urology. 1992. 40:464–467.
14. Ganem JP, Workman KR, Shaban SF. Testicular microlithiasis is associated with testicular pathology. Urology. 1999. 53:209–213.
15. Middleton WD, Teefey SA, Santillan CS. Testicular microlithiasis: prospective analysis of prevalence and associated tumor. Radiology. 2002. 224:425–428.
16. Peterson AC, Bauman JM, Light DE, McMann LP, Costabile RA. The prevalence of testicular microlithiasis in an asymptomatic population of men 18 to 35 years old. J Urol. 2001. 166:2061–2064.
17. Serter S, Gümüş B, Unlü M, Tunçyürek O, Tarhan S, Ayyildiz V, et al. Prevalence of testicular microlithiasis in an asymptomatic population. Scand J Urol Nephrol. 2006. 40:212–214.
18. Otite U, Webb JA, Oliver RT, Badenoch DF, Nargund VH. Testicular microlithiasis: is it a benign condition with malignant potential? Eur Urol. 2001. 40:538–542.
19. Ikinger U, Wurster K, Terwey B, Möhring K. Microcalcifications in testicular malignancy: diagnostic tool in occult tumor? Urology. 1982. 19:525–528.
20. Song G, Park J, Kim KS. The clinical significance of pediatric testicular microlithiasis in relation to testicular tumors. Korean J Urol. 2009. 50:57–60.
21. Konstantinos S, Alevizos A, Anargiros M, Constantinos M, Athanase H, Konstantinos B, et al. Association between testicular microlithiasis, testicular cancer, cryptorchidism and history of ascending testis. Int Braz J Urol. 2006. 32:434–438.
22. Parra BL, Venable DD, Gonzalez E, Eastham JA. Testicular microlithiasis as a predictor of intratubular germ cell neoplasia. Urology. 1996. 48:797–799.
23. Bennett HF, Middleton WD, Bullock AD, Teefey SA. Testicular microlithiasis: US follow-up. Radiology. 2001. 218:359–363.
24. Roberts IS, Loughran CF. Case report: the ultrasound appearances of testicular microlithiasis ('snow storm' testis): a case complicated by testicular seminoma. Clin Radiol. 1993. 47:65–67.
25. Sakamoto H, Shichizyou T, Saito K, Okumura T, Ogawa Y, Yoshida H, et al. Testicular microlithiasis identified ultrasonographically in Japanese adult patients: prevalence and associated conditions. Urology. 2006. 68:636–641.
26. Thomas K, Wood SJ, Thompson AJ, Pilling D, Lewis-Jones DI. The incidence and significance of testicular microlithiasis in a subfertile population. Br J Radiol. 2000. 73:494–497.
27. Skakkebaek NE, Holm M, Hoei-Hansen C, Jørgensen N, Rajpert-De Meyts E. Association between testicular dysgenesis syndrome (TDS) and testicular neoplasia: evidence from 20 adult patients with signs of maldevelopment of the testis. APMIS. 2003. 111:1–9.
28. Holm M, Lenz S, De Meyts ER, Skakkebaek NE. Microcalcifications and carcinoma in situ of the testis. BJU Int. 2001. 87:144–149.
29. Miller FN, Sidhu PS. Does testicular microlithiasis matter? A review. Clin Radiol. 2002. 57:883–890.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download