Abstract
Purpose
To analyze the preoperative clinical and pathological characteristics of patients with pT0 prostate cancer.
Materials and Methods
We retrospectively reviewed the records of 702 patients who underwent radical prostatectomy (RP) at our institution between January 2004 and July 2008 for clinically localized prostate cancer. If there was no evidence of residual tumor in the pathological specimen of the prostate, a patient was staged as pT0. Patients with pT0 disease were compared with a control group of patients who were operated on during the same period.
Results
Overall, 9 (1.3%) patients were staged as pT0 on the pathologic examination. Significant differences were observed between the pT0 group and the control patients in the biopsy Gleason score (p=0.004), the number of positive cores on biopsy (p=0.018), the tumor length of positive cores (p<0.001), and prostate volume (p=0.015). Cutoff values predictive of pT0 tumor status were defined as a biopsy Gleason score sum ≤6, 2 or fewer positive biopsy cores, tumor length on biopsy ≤2 mm, and prostate volume >30 cm3. Whereas 8 of the 9 (88.9%) pT0 patients showed all of these characteristics, only 55 of the 693 (7.9%) control patients fulfilled the criteria. The combination suggested above afforded a sensitivity of 88.8% and a specificity of 92.1% for the prediction of pT0 status.
Because of the increased use of screening tests for prostate-specific antigen (PSA), diagnosis of prostate cancer at an early clinical stage and small tumor size has recently increased, and as a consequence, tumor volume in radical prostatectomy (RP) specimens has decreased [1,2]. Pathological stage pT0 is defined as no evidence of residual tumor in an RP specimen from a patient in whom biopsy-proven prostate carcinoma was histologically diagnosed. This was termed the "vanishing cancer phenomenon" by Goldstein et al [3]. The pT0 stage of prostate cancer has been observed as a result of hormone therapy or prior transurethral resection of the prostate (TURP) for benign prostatic hyperplasia before RP [2,4-7]. Although much research has addressed these two scenarios of pT0 stage prostate cancer [8-10], patients showing pT0 status in cases other than these two scenarios occur very rarely (0.2-0.8% of all prostate cancer patients), and few studies have examined such patients [11-13].
The clinical significance of pT0 staging remains unclear. However, several reports indicate that pT0 stage patients show a highly satisfactory clinical outcome [12,13]. In a 10-year follow-up study of 38 pT0 patients, neither recurrence nor progression was observed in any patient. Therefore, to prevent unnecessary treatment of such clinically insignificant cancers, it is essential to identify preoperative clinical and pathologic characteristics that help to detect patients with a high probability of pT0 staging on RP specimens.
Because the pT0 stage of prostate cancer is rarely observed, only a few studies of the preoperative features of such patients have appeared [3,11,12,14]. Moreover, there are few reports on the characteristics of pT0 Asian patients. It has been reported that, compared to Western countries, Asian populations show a lower incidence of prostate cancer, but that Asians have high-grade prostate cancer (Gleason score above 7) and a smaller prostate volume [15-17]. Thus, considering this ethnic difference, simple adaptation of the predictive measures of prostate cancer pT0 stage used in Western countries to Asian populations may be inappropriate. Therefore, in the present study, we analyzed preoperative clinical and pathologic characteristics of patients in whom pT0 staging was confirmed after diagnosis of prostate cancer on prostate needle biopsy and RP. Patients who underwent hormone therapy or who were diagnosed with prostate carcinoma when receiving TURP for benign prostatic hyperplasia before RP were not included in this study.
We analyzed 702 patients with prostate cancer who underwent RP at our institution between January 2004 and July 2008. Those who received preoperative hormone treatment (68 cases) or who were diagnosed with prostate carcinoma by TURP for benign prostatic hyperplasia before RP (13 cases) were excluded from the analysis. All patients had clinically localized prostate cancer by digital rectal examination, endorectal ultrasonography, and magnetic resonance imaging (MRI). All patients underwent retropubic RP and pelvic lymph node dissection. We re-examined and retrospectively analyzed the following clinicopathological factors: age, PSA level, digital rectal examination result, Gleason score, number and length of positive cores in the prostate needle biopsy, and tumor volume of the RP specimen. The pathological pT0 stage was assigned when no residual tumor was seen in the RP specimen.
Each RP specimen, which was examined by two genitourinary (GU) pathology specialists at our institution, was completely fixed and cut into 3 mm slices. When areas with possible prostate cancer were observed, these regions were examined by immunochemical staining using AMACR (1:40; BIOCARE, Walnut Creek, USA) and anti-p63 (1:100; DAKO, Glostrup, Denmark). Prostate needle biopsy tissue was reexamined only when residual tumor was not observed. When prostate cancer was nevertheless detected in the biopsy during reexamination, the RP tissue block corresponding to the tumor area of the biopsy was serially sectioned and the block was turned upside down to create more serial sections. Slides were screened by pathology fellows or residents and were further examined for cancer by the two GU pathology specialists. In brief, all RP specimens of stage pT0 were examined by at least three pathologists.
Patients with pT0 stage prostate cancer were compared with a control group consisting of the remaining 693 prostate cancer patients treated at our institution during the same period. Preoperative clinical and pathologic characteristics including age, PSA level, digital rectal examination data, Gleason score, the number and length of positive cores on prostate needle biopsy, prostate volume on endorectal ultrasonography, and tumor size of the RP specimen were examined in all patients. Prostate volume was evaluated by using the ellipsoid formula, π/6 x prostate width x height x depth. These features were analyzed in a search for characteristics predictive of the pT0 stage of prostate cancer. We calculated the cutoff value of clinicopathological factors significantly correlated with pT0 staging and also investigated the sensitivity and specificity of such factors. To compare patients with pT0 prostate cancer with the control group, the chi-square test was used to analyze categorical variables (digital rectal examination), and the Mann-Whitney U test was used when consecutive variables (age, preoperative PSA level, number and length of positive cores on preoperative needle biopsy, Gleason score on needle biopsy) were examined. p-values of <0.05 were considered to reflect a statistically significant difference. SPSS, version 12.0, was used for all statistical analyses (SPSS Inc., Chicago, IL, USA).
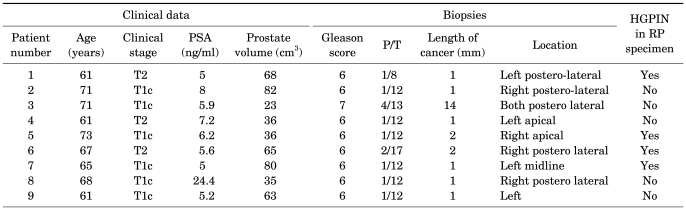
In the present study, 9 (1.3%) of the 702 patients were postoperatively diagnosed with prostate cancer of pT0 stage. The mean age of the nine patients was 66.4 years (range, 61-73 years), and their average PSA level was 8.1 ng/ml (range, 5.0-24.4 ng/ml). Of the nine patients, six (66.7%) were of clinical stage T1c, and three (33.3%) were of stage T2. Eight patients (88.9%) had a biopsy Gleason score of 6 and one patient a score of 7 (4+3). Among the eight (88.9%) patients who had two or fewer positive biopsy cores, seven had only one positive core. The other patient had two positive cores. The mean length of the positive biopsy cores was 3 mm (range, 1.0-14.0 mm). Except for the one patient in whom tumor length was 14 mm and the one patient in whom tumor length was unknown, a tumor size of 2 mm or less was found in the remaining seven patients. The mean prostate volume was 54.1 cm3 (range, 23-82 cm3), and, except for one patient (volume, 23 cm3), the prostate volume of all pT0 patients was at least 30 cm3. Of the nine patients, four had high-grade prostatic intraepithelial neoplasia (HGPIN) (Table 1). During the mean follow-up period of 23.6 months, no biochemical recurrence occurred in any patient.
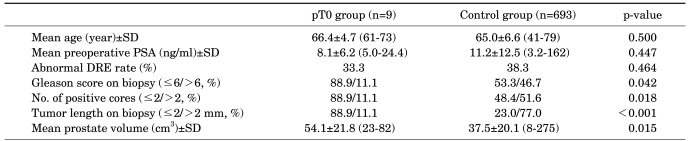
In a comparison between the two groups (the 9 patients with pT0 staging and the remaining 693 patients with tumors seen in RP specimens), no significant differences were found in mean age (66.4 vs. 65.0 years, p=0.500), PSA level (8.1 vs. 11.2 ng/ml, p=0.447), or abnormality in the digital rectal examination (33.3% vs. 38.3%, p=0.464). However, a significant difference was found when preoperative biopsy Gleason score was examined (≤6/>6; 88.9/11.1% in those with pT0 disease vs. 53.3/46.7% in the control group, p=0.042). Another significant difference was that two or fewer positive cores were seen in patients with pT0 staging (88.9% in pT0 disease vs. 48.4% in the control group, p=0.018). The size of the tumor on biopsy also differed significantly between the two groups. It was 2 mm or less in length in 88.9% of those with pT0 disease vs. 23.0% of the control group (p<0.001). Lastly, the mean prostate volume was 54.1 cm3 in pT0 patients but 37.5 cm3 in the control group, showing that pT0 patients had a larger prostate volume (p=0.015) (Table 2).
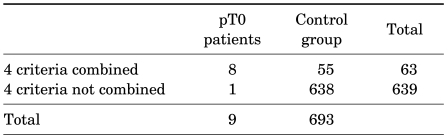
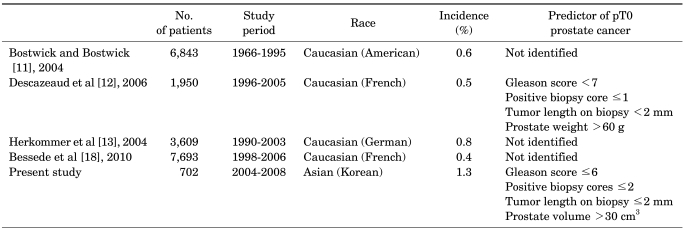
For predicting pT0 staging of prostate cancer, we choose four criteria such as a Gleason score of 6 or less, two or fewer positive cores, a tumor size of 2 mm or less on preoperative biopsy, and a prostate volume of 30 cm3. Combining the four criteria showed a sensitivity of 88.8%, a specificity of 93.4%, a positive predictive value of 12.7%, and a negative predictive value of 99.8% (Table 3). In Table 4, previous studies in the literature on the incidence and characteristics of pT0 stage patients are reviewed and compared with our results.
Previous studies have found that, after patients who received preoperative hormone therapy and who were diagnosed with prostate cancer when undergoing TURP for treatment of benign prostatic hyperplasia were excluded, the incidence of pT0 staging after RP was extremely low (0.2-0.8%) [11-13,18]. Several explanations are possible for patients being staged as pT0 after RP. First, the tumor may have been completely removed during the preoperative biopsy. A very small tumor may have been eliminated during specimen workup procedures, such as paraffin block preparation. Second, the preoperative biopsy may have yielded a false-positive result. Such false-positives can arise as a result of pathologist error. Third, the pathological examination of an RP specimen may have resulted in a false-negative finding due to a very small tumor volume, in which the tumor could be easily undetected by the pathologist, or due to an inflammatory reaction. Finally, specimen mix-up or a mislabeled specimen may result in a false-positive diagnosis of prostate cancer. DNA identity testing is available and could be used if the original diagnostic material is available [14].
Indeed, pathological examination plays an important role in diagnosing small tumors. In a study by Kollermann and colleagues, in which four GU pathology experts reexamined RP specimens from 20 pT0 patients, small residual tumors (≤0.2 ml in volume) were discovered in 13 patients [9]. In the present study, after fixation, whole specimens were cut into 3 mm slices, and when no tumor was found in these initial sections, additional tissue slides were prepared. Absence of residual tumor was confirmed by at least two GU pathology specialists.
Whereas the incidence of pT0 staging is generally known to be under 1%, the frequency was 1.3% in the present study [11-13]. Most pT0 stage patients in our study had PSA levels less than 10 ng/ml, initial prostate needle biopsies with a Gleason score of 6 or less, two or fewer positive cores, a core length of 2 mm or less, and larger tumor volume. Descazeaud et al examined 11 patients who were diagnosed as pT0 stage after RP and reported that 90.9% of the patients had PSA levels less than 15 ng/ml, 81.8% had one positive core, and all had a Gleason score of 6 or less [12]. The pT0 stage group also showed a significant difference from the control group in prostate weight (72 g vs. 51 g). Given that a small tumor is more difficult to find in a large-volume prostate, the frequency of pT0 staging after RP may be associated with prostate volume. In our analysis of preoperative characteristics in the two groups, a significant difference was found in those with a Gleason score of 6 or less, two or fewer positive cores, and core length of 2 mm or less. Also, four patients had HGPIN in the RP specimen. Mean prostate volume was also significantly larger in patients with pT0 staging than in the control group. Given that the prostate volume of Asians is smaller than that of Westerners, application of Western pT0 staging-predictive factors for prostate cancer to Asian populations is clearly inappropriate.
The use of RP for the treatment of pT0 prostate cancer is controversial; it is unclear whether RP is appropriate or excessive. In a study on patients diagnosed with T1a prostate cancer after TURP, Epstein et al claimed that a trace of disease progression within 8 years was found in only 16% of patients [19]. However, according to Carter et al, advanced prostate cancer was detected after RP in 6% of T1a prostate cancer specimens and 32% of T1b specimens [20]. It is evident that characteristics predictive of preoperative pT0 staging would be helpful in deciding whether definitive treatment should be immediately implemented or whether watchful waiting might be more appropriate.
In our analysis of the features predictive of pT0 staging after RP, the combination of a Gleason score of 6 or less, two or fewer positive cores, a positive core length of 2 mm or less, and a prostate volume of 30 cm3 or larger offered a sensitivity and a specificity of 87.5% and 93.1%, respectively. In the control group, all patients identified by these criteria had low-grade pT2 prostate cancer (Gleason score of 6 or less).
Few studies on the characteristics predictive of pT0 stage after RP have appeared. Our study is particularly meaningful in that it is the first attempt to analyze features predictive of pT0 staging in an Asian population. However, the most notable limitation of our study is the extremely low incidence of pT0 staging after RP. This low incidence is insufficient for the multiple logistic regression model that is required to investigate the predictive factors of pT0 stage after RP as in a previous study [12]. Furthermore, data on the free/total PSA ratio and PSA velocity were not available in our study, so these variables were not analyzed as predictive factors of pT0 staging after prostatectomy. Hence, further multicenter research on the value of these predictive characteristics is required.
In our study of 702 patients who underwent RP after being diagnosed with prostate carcinoma by biopsy, the rate of pT0 staging was 1.3%. We found that Gleason score, the number of positive cores, the positive core length, and the prostate volume were helpful in predicting pT0 staging of prostate cancer in RP specimens. Considering the favorable outcomes of patients with pT0 prostate cancer, these predictive factors for pT0 prostate cancer will be useful for clinicians and patients when they decide on a treatment.
References
1. Bostwick DG, Montironi R. Evaluating radical prostatectomy specimens: therapeutic and prognostic importance. Virchows Arch. 1997; 430:1–16. PMID: 9037309.

2. DiGiuseppe JA, Sauvageot J, Epstein JI. Increasing incidence of minimal residual cancer in radical prostatectomy specimens. Am J Surg Pathol. 1997; 21:174–178. PMID: 9042283.

3. Goldstein NS, Begin LR, Grody WW, Novak JM, Qian J, Bostwick DG. Minimal or no cancer in radical prostatectomy specimens. Report of 13 cases of the "vanishing cancer phenomenon". Am J Surg Pathol. 1995; 19:1002–1009. PMID: 7661273.
4. Noguchi M, Noda S, Nakashima O, Kojiro M. No residual tumor in a radical prostatectomy specimen after neoadjuvant hormonal therapy for localized prostate cancer. Oncol Rep. 2002; 9:1075–1080. PMID: 12168076.

5. Pettaway CA, Pisters LL, Troncoso P, Slaton J, Finn L, Kamoi K, et al. Neoadjuvant chemotherapy and hormonal therapy followed by radical prostatectomy: feasibility and preliminary results. J Clin Oncol. 2000; 18:1050–1057. PMID: 10694556.

6. Pisters LL, Dinney CP, Pettaway CA, Scott SM, Babaian RJ, von Eschenbach AC, et al. A feasibility study of cryotherapy followed by radical prostatectomy for locally advanced prostate cancer. J Urol. 1999; 161:509–514. PMID: 9915437.

7. Schulman CC. Neoadjuvant androgen blockade prior to prostatectomy: a retrospective study and critical review. Prostate Suppl. 1994; 5:9–14. PMID: 7513532.

8. Köllermann J, Caprano J, Budde A, Weidenfeld H, Weidenfeld M, Hopfenmüller W, et al. Follow-up of nondetectable prostate carcinoma (pT0) after prolonged PSA-monitored neoadjuvant hormonal therapy followed by radical prostatectomy. Urology. 2003; 62:476–480. PMID: 12946750.

9. Köllermann J, Feek U, Müller H, Kaulfuss U, Oehler U, Helpap B, et al. Nondetected tumor (pT0) after prolonged, neoadjuvant treatment of localized prostatic carcinoma. Eur Urol. 2000; 38:714–720. PMID: 11111189.
10. Köllermann J, Hopfenmüller W, Caprano J, Budde A, Weidenfeld H, Weidenfeld M, et al. Prognosis of stage pT0 after prolonged neoadjuvant endocrine therapy of prostate cancer: a matched-pair analysis. Eur Urol. 2004; 45:42–45. PMID: 14667514.
11. Bostwick DG, Bostwick KC. 'Vanishing' prostate cancer in radical prostatectomy specimens: incidence and long-term follow-up in 38 cases. BJU Int. 2004; 94:57–58. PMID: 15217431.

12. Descazeaud A, Zerbib M, Flam T, Vieillefond A, Debre B, Peyromaure M. Can pT0 stage of prostate cancer be predicted before radical prostatectomy? Eur Urol. 2006; 50:1248–1252. PMID: 16828964.

13. Herkommer K, Kuefer R, Gschwend JE, Hautmann RE, Volkmer BG. Pathological T0 prostate cancer without neoadjuvant therapy: clinical presentation and follow-up. Eur Urol. 2004; 45:36–41. PMID: 14667513.

14. Montironi R, Cheng L, Lopez-Beltran A, Scarpelli M, Mazzucchelli R, Mikuz G, et al. Stage pT0 in radical prostatectomy with no residual carcinoma and with a previous positive biopsy conveys a wrong message to clinicians and patients: why is cancer not present in the radical prostatectomy specimen? Eur Urol. 2009; 56:272–274. PMID: 19443101.

15. Oesterling JE, Kumamoto Y, Tsukamoto T, Girman CJ, Guess HA, Masumori N, et al. Serum prostate-specific antigen in a community-based population of healthy Japanese men: lower values than for similarly aged white men. Br J Urol. 1995; 75:347–353. PMID: 7537604.

16. Song C, Ro JY, Lee MS, Hong SJ, Chung BH, Choi HY, et al. Prostate cancer in Korean men exhibits poor differentiation and is adversely related to prognosis after radical prostatectomy. Urology. 2006; 68:820–824. PMID: 17070360.

17. Whittemore AS. Prostate cancer. Cancer Surv. 1994; 19-20:309–322. PMID: 7895221.
18. Bessede T, Soulie M, Mottet N, Rebillard X, Peyromaure M, Ravery V, et al. Stage pT0 after radical prostatectomy with previous positive biopsy sets: a multicenter study. J Urol. 2010; 183:958–962. PMID: 20083270.
19. Epstein JI, Paull G, Eggleston JC, Walsh PC. Prognosis of untreated stage A1 prostatic carcinoma: a study of 94 cases with extended followup. J Urol. 1986; 136:837–839. PMID: 3761442.

20. Carter HB, Kettermann A, Warlick C, Metter EJ, Landis P, Walsh PC, et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J Urol. 2007; 178:2359–2364. PMID: 17936806.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download