Abstract
Purpose
Korean red ginseng (KRG) is a potent antioxidant and a free radical scavenger. This study was designed to determine whether KRG could protect against dysfunction and oxidative stress induced by torsion-detorsion injury in rat testis.
Materials and Methods
Six-week-old male Sprague-Dawley rats were randomly divided into four groups: a sham-operated control group (C), a sham-operated and KRG-treated group (K), a 2 hours torsion and detorsion group (T), and a 2 hours torsion and detorsion and KRG-treated group (T+K). We measured testis weight and hormone levels and reactive oxygen species (ROS) from the left renal vein. Superoxide generation was measured on the basis of lucigenin-enhanced chemiluminescence in testis tissue.
Results
Testicular weight was significantly higher in the T+K group than in the T group; however, there were no significant differences in hormone levels between the 4 groups. The mean level of ROS and superoxide production was significantly higher in the T group than in the C group, whereas administration of KRG attenuated this increase. Upon histologic evaluation, the T group was found to have cellular disarray, a lack of cellular cohesiveness, degenerative changes in the germinal cells, and less distinct changes in the seminiferous tubules, whereas the T+K group had a germinal epithelial layer that appeared nearly normal.
Testicular torsion is a urologic emergency. The testicular salvage rate following surgical detorsion has been reported to range from 42% to 88% [1,2], but it is unclear whether these testes were actually saved with respect to testicular spermatogenic function [3]. A testis injury produced by reperfusion can be more severe than that induced by ischemia [4]. This is because the reperfusion component typically involves the generation of toxic reactive oxygen species (ROS) with the return of blood flow following a period of ischemia [5].
Panax ginseng (P. ginseng) C. A. Mayer has been used for thousands of years in Asia due to its wide spectrum of medicinal effects. This compound has a wide range of pharmacologic and physiologic actions, such as anti-aging, immunoenhancement, anti-stress, and anti-tumor activity [6-8]. It has long been known that P. ginseng has protective properties against free radical attack [9-11]. Indeed, P. ginseng administration to rats has been shown to prevent myocardial ischemia-reperfusion (I-R) damage induced by hyperbaric oxygen [11]. Protective effects of P. ginseng against hepatic oxidative stress induced by exhaustive exercise and against skeletal muscle injury caused by oxidative stress induced by acute exercise have also been reported [12,13]. Among the currently available P. ginseng products, Korean red ginseng (KRG, Ginseng Radix Rubra) has the most potent multiple pharmacologic actions for anticancer, antihypertension, antidiabetes, and anti-nociception effects and for improving weak body conditions [14]. Recently, KRG was also found to possess anti-stress and antioxidant activities [15].
Although KRG has been investigated for multiple purposes, its effect on I-R injury of the testes during testicular torsion and detorsion has not yet been evaluated. In the present study, we aimed to evaluate the effects of KRG on testicular damage in a rat testicular I-R injury model.
Steamed ginseng (KRG, Cheong-Kwan-Jang) was purchased from the Korea Ginseng Corporation (Daejeon, Korea) and dissolved in distilled water. KRG (100 mg/kg per day) was administered orally for 4 weeks after surgery and continued until the end of the experiment.
Forty 6-week-old male Sprague-Dawley rats (150±25 g, Jung-ang, Inc., Daejeon, Korea) were randomly divided into four groups: a sham-operated group (C), a sham-operated and KRG-treated group (K), a 2 hours torsion and detorsion group (T), and a 2 hours torsion and detorsion and KRG-treated group (T+K). The sham-operated group was used as the baseline group. All animals underwent an adaptation period of 1 week in cages under normal conditions before the surgical procedure. At the age of 7 weeks, the C and K groups underwent a sham operation and the T and T+K groups underwent testicular torsion followed by detorsion. The C and T groups were fed tap water, whereas the K and T+K groups were fed water with KRG for 4 weeks. After 4 weeks, the rats were anesthetized and the left testes were removed. After the testes were removed, they were dissected to remove the fat tissue and blood vessels, after which the tunica albuginea was eliminated. The testis was then weighed and longitudinally bisected. Half of the testes were immediately stored in organ baths to determine the superoxide level, whereas the remainder were fixed in Bouin's solution for histological examination. Fresh heparinized blood samples from the left renal vein were also obtained to assess the antioxidant enzyme, ROS, and hormonal levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone. The remainder of the testis was stored at -70℃ for biochemical assay.
Male Sprague-Dawley rats (160±20 g, 7 weeks old) were fasted for 24 hours before the experiments, but were provided with tap water. In all protocols, a combination of 80 mg/kg ketamine (Huons Corp. Seoul, Korea) and 12 mg/kg xylazine (Bayer Health Care, Germany) was used to induce anesthesia. All operations were conducted under sterile conditions. Throughout the anesthesia, body temperature was maintained by using a heating pad. In the sham-operated group, the left testis was removed through a midscrotal vertical incision. A 5-0 silk suture was then placed through the tunica albuginea, the left testis was placed into the scrotum, and the incision was closed. In the torsion and detorsion group, the left testis was exposed through the same incision. The left testis was rotated 720° in a counterclockwise direction and maintained in this torsion position by fixing the testis to the scrotum with a 5-0 silk suture. After 2 hours of torsion, the testis was counter-rotated to the natural position.
After 4 weeks of KRG administration, we measured the left testis weight.
Blood was collected from the left renal vein into a bottle and the levels of FSH, LH, and testosterone were then measured at a laboratory (Sam-kwang, Seoul, Korea).
All tissue specimens were kept in Bouin's solution. Following the fixation procedure, tissue specimens were embedded in paraffin, sectioned at a thickness of 5 µm, and then stained with H&E.
A lucigenin-enhanced chemiluminescence assay was used to measure the level of superoxide production as previously reported [16,17]. Lucigenin (bis-N-methylacridinium nitrate) luminescens specifically in the presence of superoxide. Dark-adapted lucigenin solution (5 µmol/l) was prepared in aerated Krebs-HEPES buffer (NaCl 100 mmol/l, KCl 4.7 mmol/l, CaCl2 1.9 mmol/l, MgSO4 1.2 mmol/l, K2HPO4 1.03 mmol/l, NaHCO3 25 mmol/l, Na-HEPES 20 mmol/l, pH 7.4). The testis parenchyme was then transferred into scintillation vials containing Krebs-HEPES buffer with 5 µM lucigenin. The chemiluminescence, which occurred over the ensuing 2 minutes in response to the addition of 100 µM NADPH, was then recorded. After subtraction of the value of a blank, the emitted light units were used as a measure of superoxide production. Values are expressed as relative light units per 1 µg of tissue (RLU/1 µg tissue).
The blood levels of ROS were determined by using the free oxygen radical test (FORT). To conduct the FORT, blood samples from the left renal vein were immediately thawed and mixed with an acidic buffer that contained transition metals. The radical species produced by the reaction is the area directly proportional to the quantity of lipid peroxides present in the sample that interacts with an additive (phenylenediamine derivative) to form a radical molecule that can be detected at 505 nm by a spectrophotometer (Form CR 2000, Callegari, Parma, Italy). The intensity of the color corresponds directly to the quantity of peroxy and alkoxy radicals that form upon addition of the transition metals. All results are expressed as FORT units, where 1 FORT unit corresponds to 0.26 mg/l of H2O2. Higher levels of FORT indicate higher oxidative stress levels.
Data are expressed as the mean±standard error of mean (SEM). SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA) was used to evaluate the data. One-way and two-way ANOVAs were used to evaluate the hormonal levels. The Mann-Whitney method analysis of variance was used to evaluate the other parameters. Values of p<0.05 were considered to be statistically significant.
The testicular weights of groups C and K were significantly different from the weights of groups T and T+K. In addition, although there were no significant differences in the testicular weights of groups C and K, the testicular weights of groups T and T+K differed significantly (Fig. 1).
There were no significant differences in the hormone levels (FSH, LH, and testosterone) of the 4 groups (Table 1).
The normal appearance of the testicular structure of rats was determined by evaluation of group C (Fig. 2A), which had a normal arrangement of germinative cells in the seminiferous tubules. The appearance of group K (Fig. 2B) was similar to that of group C. However, group T (Fig. 2C) showed cellular disarray, a lack of cellular cohesiveness, degenerative changes in the germinal cells, and less distinct changes in the seminiferous tubule. In the T+K group (Fig. 2D), the germinal epithelial layer appeared nearly normal.
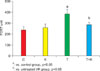
The basal testis superoxide was quantified by lucigenin-enhanced chemiluminescence. The superoxide levels were significantly higher in the T group (278.3±39.6 for the T group vs. 181±16.8 for the C group and 189.5±13.2 for the K group; all values RLU/1 mg tissue, p<0.05). The superoxide level decreased significantly in response to treatment with KRG (173.3±13.0 RLU/1 mg tissue, p<0.05). NADPH-driven superoxide production was markedly increased in the T group (Fig. 3), whereas it was significantly decreased by treatment with KRG.
As shown in Fig. 4, the mean level of FORT, which is an oxidative stress marker, was significantly higher in the T group than in the C group (386.2±43.9 FORT units vs. 240.5±27.9 FORT units, p<0.05). KRG administration attenuated the increase in the testis FORT level (286.0±26.8 FORT units, p<0.05).
Testicular torsion is the twisting of the spermatic cord, which cuts off the blood supply to the testicle and surrounding structures within the scrotum. Testicular torsion is a very common surgical emergency [18]. If surgery is performed within hours, most testicles can be saved, although there is an increased risk of decreased sperm production and fertility and atrophy of the testis [19,20]. Testicular torsion-detorsion is an I-R injury to the testis. In this study, we evaluated KRG treatment to determine whether it could protect the testis from injury associated with testicular torsion and detorsion. To accomplish this, we measured the superoxide level of the I-R damaged testis tissue with and without KRG treatment. This study is the first report to evaluate the effects of KRG, a potent antioxidant, on the formation of ROS in rat testis due to I-R injury. The results of the study demonstrated that KRG recovered the testis dysfunction caused by ischemia and subsequent reperfusion in the rat testis by suppressing superoxide production.
It has been suggested that many substances are important to the prevention of testis dysfunction secondary to ischemia and reperfusion. Unsal et al reported the protective effects of garlic extract on rat testis subjected to a torsion and detorsion model [21]. In their study, the authors concluded that the effect of garlic extract appeared to occur through inhibition of the xanthine oxidase-mediated I-R injury cascade. Cay et al reported that N-acetylcysteine may be a useful agent for preventing the oxidative effects of reperfusion injury following torsion [22]. Additionally, Lim et al reported that pretreatment with allopurinol prevented I-R injury in testis [23].
Among the components of P. ginseng, KRG has been shown to exhibit a variety of antioxidative actions. Kitts et al reported that North American ginseng extract exhibits effective antioxidant activity in both lipid and aqueous media via the chelation of metal ions and scavenging of free radicals [24]. Kim et al reported that red ginseng aqueous extract had free radical scavenging activity [25]. Indeed, red ginseng extracts are known to scavenge hydroxyl and superoxide radicals. P. ginseng has been shown to have a remarkable capacity to protect brain tissue proteins from oxidative damage in vitro [26]. Furthermore, the crude saponin fraction of KRG was found to abrogate the generation of NADPH-driven superoxide in rats [15]. Overall, the aforementioned studies indicate that KRG is a useful adjuvant for the protection of I-R-induced oxidative injury. Therefore, it is likely that KRG also has protective effects against I-R-induced oxidative injury of the testis, which was confirmed in the present study.
Ischemia is not an isolated event, and when reversible, it is followed by reperfusion. Reperfusion may cause a more severe injury than ischemia alone [8]. I-R injury is associated with the over-generation of ROS, such as hypochlorous acid, nitric oxide, hydrogen peroxide, superoxide anion, and hydroxyl radicals [3]. ROS cause damage to DNA and impair the protein function and peroxidation of lipids. Lipid peroxides rupture the membranes and cause structural and functional alterations in the testis. Reperfusion of the ischemic tissue promotes the generation of ROS, which arises from activation of the xanthine oxidase system in parenchymal cells or from leukocytes penetrating into interstitial tissue. These substances have destructive effects on various cellular functions and lead to increased microvascular permeability, interstitial edema, impaired vasoregulation, inflammatory cell infiltration, and parenchymal cell dysfunction and necrosis. Therefore, ROS may directly damage the seminiferous tubules in the testis. Free radical reduction for the treatment of I-R injury has been found to prevent post-ischemic testis dysfunction [27].
The FORT provides an indirect measure of hydroperoxides, which are a useful measure of oxidative stress because they indicate the presence of intermediate oxidative products of lipids, amino acids, and peptides. Increases in FORT values most likely indicate an increase in the oxidation of lipids, which occurs in lipid bilayers and lipid particles [28]. This oxidative marker also reflects specific cellular oxidation events. Abramson et al suggested that oxidative stress, as measured by the free radical oxygen test, may be a determinant of C-reactive protein levels and promote pro-atherosclerotic inflammatory processes [29]. In the present study, the correlation between FORT and the acute-phase reactant C-reactive protein was evaluated and a significant positive correlation was found. Increasing FORT values also indicate inflammatory processes. I-R led to increased oxidative stress in the testis, but KRG had a protective effect against the oxidation of lipid bilayers and inflammatory processes.
Among various sources of superoxide such as NADPH oxidase, xanthine oxidase, lipoxygenase, mitochondrial oxidase, and nitric oxide synthase, NADPH oxidase appears to be the principal source of superoxide in several animal models. In the present study, KRG inhibited basal superoxide production and NADPH oxidase in the testis. KRG inhibits the generation of reactive oxygen species that damage the cellular membranes. Post-treatment with KRG protects this continuing membrane damage that underlies progressive testis dysfunction.
In conclusion, KRG is a potent antioxidant agent that protects against oxidative damage to the testis tissue. KRG treatment prevents testis dysfunction via decreased production of NADPH oxidase and superoxide. Based on the results of this study, we suggest that KRG may play a role in the protection of I-R-induced oxidant injury in the rat testis. However, in vivo and in vitro studies are required to determine the exact role that KRG plays in the protection of the testes against I-R injury.
The results of the present study demonstrated that I-R leads to testis dysfunction, as well as the induction of ROS. The present study demonstrated that KRG recovered the testis dysfunction caused by ischemia and subsequent reperfusion in the rat testis through the suppression of superoxide production. Further studies are needed to explain the mechanisms of protection.
Figures and Tables
FIG. 1
Weight of the testis. C: a sham-operated group, K: a sham-operated and KRG-treated group, T: a 2 hours torsion and detorsion group, T+K: a 2 hours torsion and detorsion and KRG-treated group.

FIG. 2
Representative microphotographs of cross-sections of the seminiferous tubules from SD rats. (A: group C, B: group K, C: group T, D: group T+K) (H&E, ×400). C: a sham-operated group, K: a sham-operated and KRG-treated group, T: a 2 hours torsion and detorsion group, T+K: a 2 hours torsion and detorsion and KRG-treated group.
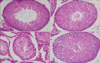
FIG. 3
Lucigenin-enhanced chemiluminescence, reflective of testis superoxide levels. Luminescence was recorded in the absence or presence of NADPH (100 µmol/l). C: a sham-operated group, K: a sham-operated and KRG-treated group, T: a 2 hours torsion and detorsion group, T+K: a 2 hours torsion and detorsion and KRG-treated group.

References
1. Barada JH, Weingarten JL, Cromie WJ. Testicular salvage and age-related delay in the presentation of testicular torsion. J Urol. 1989. 142:746–748.
2. Cattolica EV, Karol JB, Rankin KN, Klein RS. High testicular salvage rate in torsion of the spermatic cord. J Urol. 1982. 128:66–68.
3. Wei SM, Yan ZZ, Zhou J. Beneficial effect of taurine on testicular ischemia-reperfusion injury in rats. Urology. 2007. 70:1237–1242.
4. Unsal A, Devrim E, Guven C, Eroglu M, Durak I, Bozoklu A, et al. Propofol attenuates reperfusion injury after testicular torsion and detorsion. World J Urol. 2004. 22:461–465.
5. Power RE, Scanlon R, Kay EW, Creagh TA, Bouchier-Hayes DJ. Long-term protective effects of hypothermia on reperfusion injury post-testicular torsion. Scand J Urol Nephrol. 2003. 37:456–460.
6. Sugaya A, Yuzurihara M, Tsuda T, Yasuda K, Kajiwara K, Sugaya E. Proliferative effect of ginseng saponin on neurite extension of primary cultured neurons of the rat cerebral cortex. J Ethnopharmacol. 1988. 22:173–181.
7. Hasegawa H, Suzuki R, Nagaoka T, Tezuka Y, Kadota S, Saiki I. Prevention of growth and metastasis of murine melanoma through enhanced natural-killer cytotoxicity by fatty acid-conjugate of protopanaxatriol. Biol Pharm Bull. 2002. 25:861–866.
8. Kaneko H, Nakanishi K. Proof of the mysterious efficacy of ginseng: basic and clinical trials: clinical effects of medical ginseng, Korean red ginseng: specifically, its anti-stress action for prevention of disease. J Pharmacol Sci. 2004. 95:158–162.
9. Chen X. Cardiovascular protection by ginsenosides and their nitric oxide releasing action. Clin Exp Pharmacol Physiol. 1996. 23:728–732.
10. Zhu D, Wu L, Li CR, Wang XW, Ma YJ, Zhong ZY, et al. Ginsenoside Rg1 protects rat cardiomyocyte from hypoxia/reoxygenation oxidative injury via antioxidant and intracellular calcium homeostasis. J Cell Biochem. 2009. 108:117–124.
11. Maffei Facino R, Carini M, Aldini G, Berti F, Rossoni G. Panax ginseng administration in the rat prevents myocardial ischemia-reperfusion damage induced by hyperbaric oxygen: evidence for an antioxidant intervention. Planta Med. 1999. 65:614–619.
12. Voces J, Alvarez AI, Vila L, Ferrando A, Cabral de Oliveira C, Prieto JG. Effects of administration of the standardized Panax ginseng extract G115 on hepatic antioxidant function after exhaustive exercise. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999. 123:175–184.
13. Voces J, Cabral de Oliveira AC, Prieto JG, Vila L, Perez AC, Duarte ID, et al. Ginseng administration protects skeletal muscle from oxidative stress induced by acute exercise in rats. Braz J Med Biol Res. 2004. 37:1863–1871.
14. Kaneko H, Nakanishi K. Proof of the mysterious efficacy of ginseng: basic and clinical trials: clinical effects of medical ginseng, korean red ginseng: specifically, its anti-stress action for prevention of disease. J Pharmacol Sci. 2004. 95:158–162.
15. Kim CS, Park JB, Kim KJ, Chang SJ, Ryoo SW, Jeon BH. Effect of Korea red ginseng on cerebral blood flow and superoxide production. Acta Pharmacol Sin. 2002. 23:1152–1156.
16. Skatchkov MP, Sperling D, Hink U, Mülsch A, Harrison DG, Sindermann I, et al. Validation of lucigenin as a chemiluminescent probe to monitor vascular superoxide as well as basal vascular nitric oxide production. Biochem Biophys Res Commun. 1999. 254:319–324.
17. Jeon BH, Gupta G, Park YC, Qi B, Haile A, Khanday FA, et al. Apurinic/apyrimidinic endonuclease 1 regulates endothelial NO production and vascular tone. Circ Res. 2004. 95:902–910.
18. Yazihan N, Ataoglu H, Koku N, Erdemli E, Sargin AK. Protective role of erythropoietin during testicular torsion of the rats. World J Urol. 2007. 25:531–536.
19. Horica CA, Hadziselimovic F, Kreutz G, Bandhauer K. Ultrastructural studies of the contorted and contralateral testicle in unilateral testicular torsion. Eur Urol. 1982. 8:358–362.
20. Thomas WE, Williamson RC. Diagnosis and outcome of testicular torsion. Br J Surg. 1983. 70:213–216.
21. Unsal A, Eroglu M, Avci A, Cimentepe E, Guven C, Derya Balbay M, et al. Protective role of natural antioxidant supplementation on testicular tissue after testicular torsion and detorsion. Scand J Urol Nephrol. 2006. 40:17–22.
22. Cay A, Alver A, Kücük M, Isik O, Eminagaoglu MS, Karahan SC, et al. The effects of N-acetylcysteine on antioxidant enzyme activities in experimental testicular torsion. J Surg Res. 2006. 131:199–203.
23. Lim DJ, Hong SK, Jeong SJ, Choi H. The mechanism of damage to the contralateral testis following testicular torsion and detorsion in rats and the effect of allopurinol administration. Korean J Urol. 2006. 47:180–188.
24. Kitts DD, Wijewickreme AN, Hu C. Antioxidant properties of a North American ginseng extract. Mol Cell Biochem. 2000. 203:1–10.
25. Kim YK, Guo Q, Packer L. Free radical scavenging activity of red ginseng aqueous extracts. Toxicology. 2002. 172:149–156.
26. Siddique MS, Eddeb F, Mantle D, Mendelow AD. Extracts of Ginkgo biloba and Panax ginseng protect brain proteins from free radical induced oxidative damage in vitro. Acta Neurochir Suppl. 2000. 76:87–90.
27. Granger DN, Korthuis RJ. Physiologic mechanisms of postischemic tissue injury. Annu Rev Physiol. 1995. 57:311–332.
28. Mercuro G, Cadeddu C, Piras A, Dessi M, Madeddu C, Deidda M, et al. Early epirubicin-induced myocardial dysfunction revealed by serial tissue Doppler echocardiography: correlation with inflammatory and oxidative stress markers. Oncologist. 2007. 12:1124–1133.
29. Abramson JL, Hooper WC, Jones DP, Ashfaq S, Rhodes SD, Weintraub WS, et al. Association between novel oxidative stress markers and C-reactive protein among adults without clinical coronary heart disease. Atherosclerosis. 2005. 178:115–121.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download