Abstract
Purpose
The radiobiology of prostate cancer favors a hypofractionated dose regimen. We report here our experience with the CyberKnifeTM, demonstrating its efficacy, safety, and feasibility as a treatment modality for non-metastatic prostate cancer.
Materials and Methods
Between October 2002 and April 2006, 20 patients with biopsy-proven prostate cancer were treated with the CyberKnifeTM. The distribution of clinical risks, as assessed by using D'Amico's definition for risk grouping, was as follows: low (4), intermediate (5), and high (11). Three patients received 32 Gy, 7 patients received 34 Gy, and 10 patients received 36 Gy. All patients received the radiation doses in 4 fractions. The rectal and bladder toxicities were graded by using the criteria set forth by the Radiation Therapy Oncology Group (RTOG).
Results
The mean patient age was 71.4 years (range, 52-79 years), and the mean followup period was 35.5 months (range, 8-74 months). There were 2 acute and 1 late grade 2 gastrointestinal toxicities, and 1 acute and 2 late grade 2 urinary toxicities. The 5-year overall survival rate was 100%, respectively. The 5-year biochemical failure-free rate of the low-risk, intermediate-risk, and high-risk patients was 100%, 100%, and 90.9%, respectively.
Conclusions
CyberKnifeTM is a safe, well-tolerated, and rather effective treatment for non-metastatic prostate cancer. We obtained a 100% 5-year biochemical failure-free rate in low-risk and intermediate-risk patients. CyberKnifeTM is a viable option for the treatment of non-metastatic prostate cancer.
REFERENCES
1. Annual Report of the Korea Central Cancer Registry (based on registered data from 134 hospitals). Korea central cancer registry, ministry of health and welfare republic of Korea. 2003.
2. Grills IS, Martinez AA, Hollander M, Huang R, Goldman K, Chen PY, et al. High dose rate brachytherapy as prostate cancer monotherapy reduces toxicity compared to low dose rate palladium seeds. J Urol. 2004; 171:1098–104.

3. Horwitz EM, Hanlon AL, Pinover WH, Anderson PR, Hanks GE. Defining the optimal radiation dose with three-dimensional conformal radiation therapy for patients with nonmetastatic prostate carcinoma by using recursive partitioning techniques. Cancer. 2001; 92:1281–7.

4. Pollack A, Zagars GK, Smith LG, Lee JJ, von Eschenbach AC, Antolak JA, et al. Preliminary results of a randomized radiotherapy dose-escalation study comparing 70 Gy with 78 Gy for prostate cancer. J Clin Oncol. 2000; 18:3904–11.

5. Zelefsky MJ, Leibel SA, Gaudin PB, Kutcher GJ, Fleshner NE, Venkatramen ES, et al. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys. 1998; 41:491–500.

6. King CR, Lehmann J, Adler JR, Hai J. CyberKnife radiotherapy for localized prostate cancer: rationale and technical feasibility. Technol Cancer Res Treat. 2003; 2:25–30.

7. Adler JR Jr, Chang SD, Murphy MJ, Doty J, Geis P, Hancock SL. The Cyberknife: a frameless robotic system for radiosurgery. Stereotact Funct Neurosurg. 1997; 69:124–8.

8. Kim GH, Park K, Jo MK, Lee CW, Yang KM, Cho CG. CyberKnife for the treatment of nonmetastatic prostate cancer: preliminary results. Korean J Urol. 2006; 47:1172–7.

9. D'Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. J Clin Oncol. 2003; 21:2163–72.
10. Abramowitz MC, Li T, Buyyounouski MK, Ross E, Uzzo RG, Pollack A, et al. The Phoenix definition of biochemical failure predicts for overall survival in patients with prostate cancer. Cancer. 2008; 112:55–60.

11. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995; 31:1341–6.

12. Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: longterm results. J Urol. 2004; 172:910–4.

13. Zelefsky MJ, Chan H, Hunt M, Yamada Y, Shippy AM, Amols H. Longterm outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol. 2006; 176:1415–9.

14. Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003; 169:517–23.

15. Sylvester JE, Grimm PD, Blasko JC, Millar J, Orio PF 3rd, Skoglund S, et al. 15-year biochemical relapse free survival in clinical Stage T1-T3 prostate cancer following combined external beam radiotherapy and brachytherapy; Seattle experience. Int J Radiat Oncol Biol Phys. 2007; 67:57–64.

16. Oh MM, Bahk YW, Tropper SE. Preliminary report of clinical experience of iodine-125 seed implant for early prostatic cancer: the first case in Korea. Korean J Urol. 2001; 42:1235–40.
17. Webb S. Optimizing the planning of intensity-modulated radiotherapy. Phys Med Biol. 1994; 39:2229–46.

18. Jang J, Lee DH, Kang YN, Shin DO, Kim MC, Yoon SC, et al. The development of quality assurance program for CyberKnife. J Korean Soc Ther Radiol Oncol. 2006; 24:185–91.
19. King CR, Lehmann J, Adler JR, Hai J. CyberKnife radiotherapy for localized prostate cancer: rationale and technical feasibility. Technol Cancer Res Treat. 2003; 2:25–30.

20. Roach M 3rd, Hanks G, Thames H Jr, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006; 65:965–74.

21. Lee WR, Bae K, Lawton C, Gillin M, Morton G, Firat S, et al. Late toxicity and biochemical recurrence after external-beam radiotherapy combined with permanent-source prostate brachytherapy: analysis of Radiation Therapy Oncology Group study 0019. Cancer. 2007; 109:1506–12.
22. Ishikawa H, Tsuji H, Kamada T, Yanagi T, Mizoe JE, Kanai T, et al. Carbon ion radiation therapy for prostate cancer: results of a prospective phase II study. Radiother Oncol. 2006; 81:57–64.

23. Park W, Huh SJ, Choi HY, Lee HM, Chai SE, Ahn YC, et al. Results of definitive radiotherapy in the treatment of prostate cancer. Korean J Urol. 2005; 46:221–8.
24. Albertsen PC, Hanley JA, Penson DF, Barrows G, Fine J. 13-year outcomes following treatment for clinically localized prostate cancer in a population based cohort. J Urol. 2007; 177:932–6.

25. Hancock SL, Cox RS, Bagshaw MA. Prostate specific antigen after radiotherapy for prostate cancer: a reevaluation of longterm biochemical control and the kinetics of recurrence in patients treated at Stanford University. J Urol. 1995; 154:1412–7.
Fig. 1.
CyberKnifeTM treatment planning for prostate and critical organs in the Accuray multiplan system.
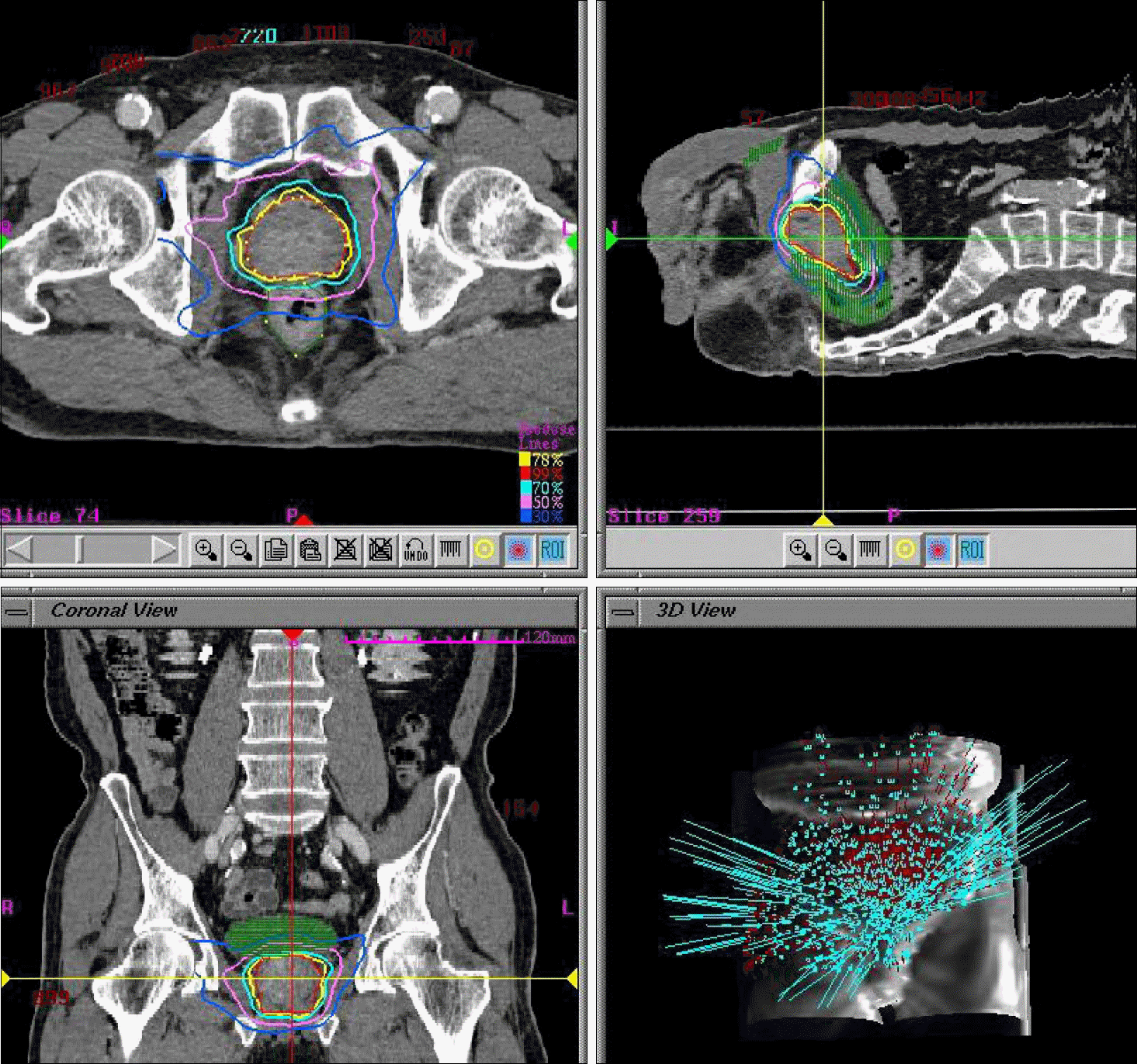
Fig. 2.
Prostate-specific antigen (PSA) response plotted as mean PSA as a function of time after CyberKnifeTM treatment. The error bar indicates ±1 SD from the mean. SD: standard deviation, CK: CyberKnifeTM.
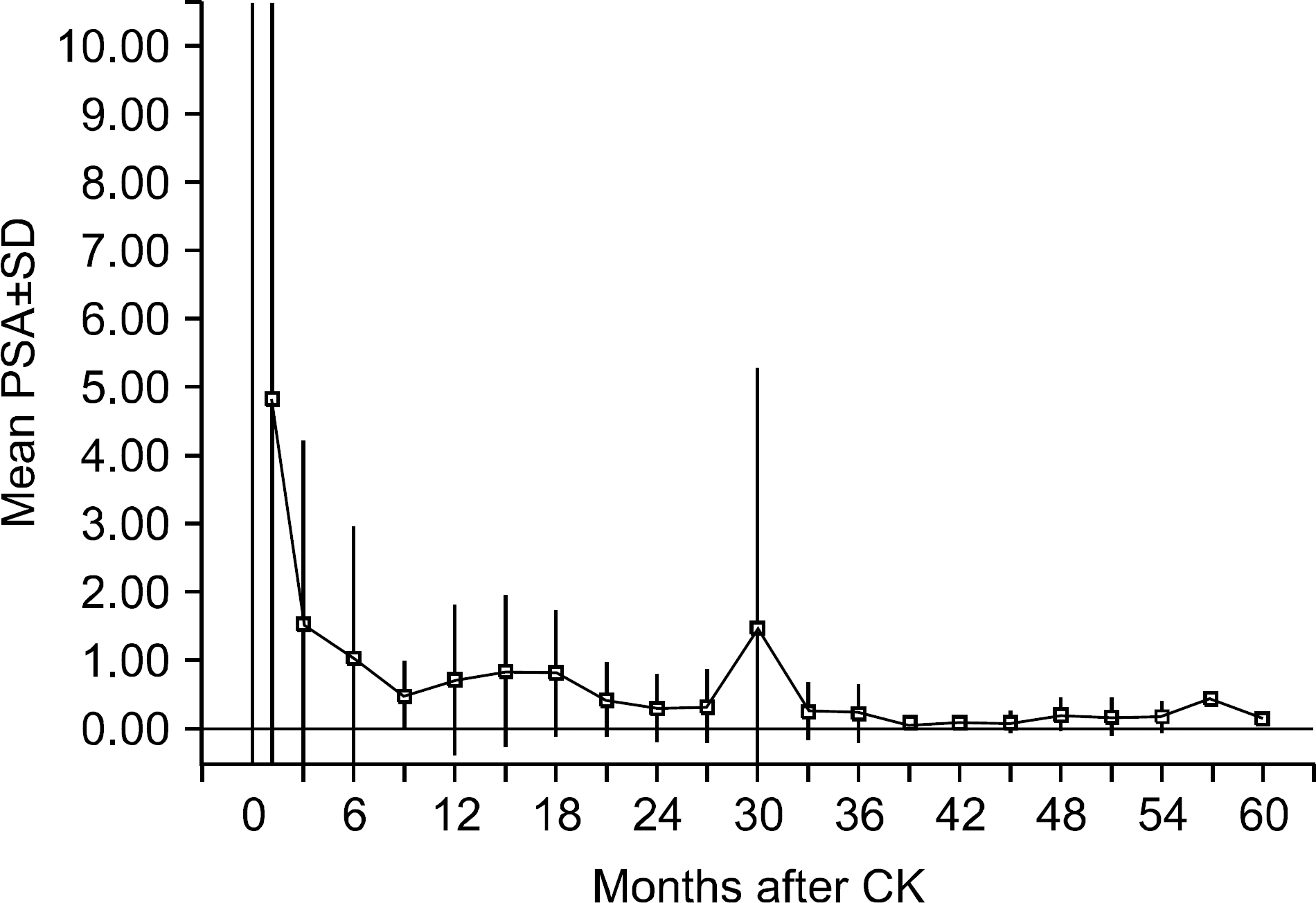
Fig. 3.
Kaplan-Meier actuarial probability of the biochemical failure-free survival rate according to risk group. CK: CyberKnifeTM.
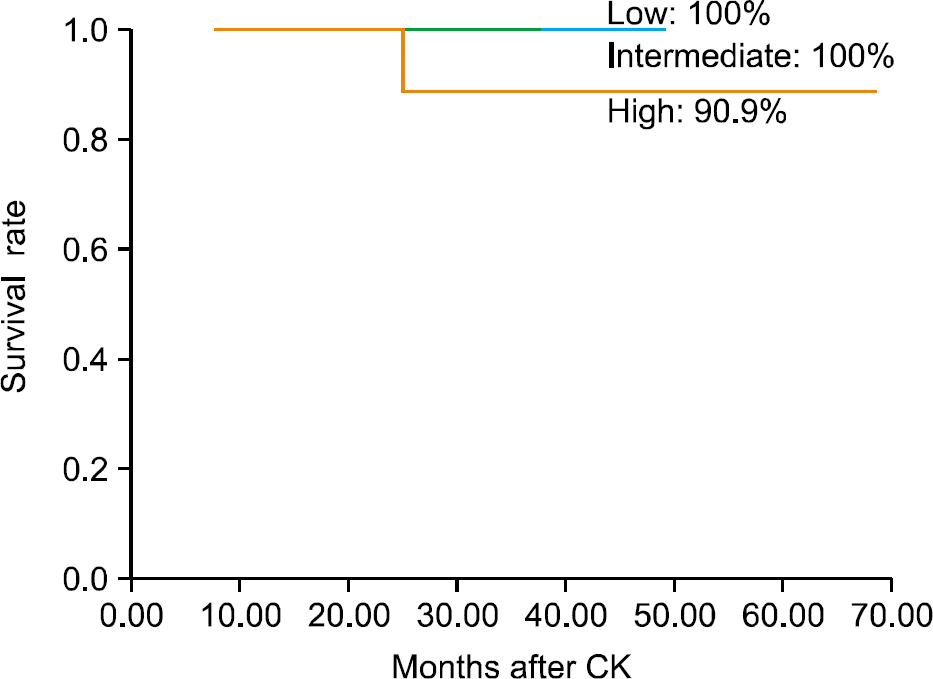
Table 1.
Clinical characteristics of the 20 consecutive prostate cancer patients treated with the CyberKnifeTM
| Case number | Age (years) | Pre-treatment PSA | Gleason score | Clinical stage | Prognostic groupsa |
|---|---|---|---|---|---|
| Non-hormonal treatment group | |||||
| 1 | 70 | 7.5 | 3+3 | 2a | Low |
| 2 | 79 | 5.4 | 3+3 | 1c | Low |
| 3 | 68 | 7.7 | 2+2 | 1c | Low |
| 4 | 78 | 1.7 | 3+3 | 2a | Low |
| 5 | 73 | 14.9 | 2+2 | 2a | Intermediate |
| 6 | 74 | 11.9 | 2+2 | 2a | Intermediate |
| 7 | 65 | 11.0 | 3+3 | 1c | Intermediate |
| 8 | 77 | 19.6 | 4+3 | 1c | Intermediate |
| 9 | 75 | 10.5 | 4+3 | 1c | Intermediate |
| Hormonal treatment group | |||||
| 10 | 76 | 4.0 | 4+4 | 2a | High |
| 11 | 76 | 29.9 | 1+1 | 2a | High |
| 12 | 77 | 19.6 | 4+5 | 3a | High |
| 13 | 70 | 115.0 | 4+4 | 3a | High |
| 14 | 66 | 40.9 | 2+2 | 2c | High |
| 15 | 75 | 78.6 | 4+3 | 1c | High |
| 16 | 69 | 38.0 | 3+3 | 2b | High |
| 17 | 75 | 20.6 | 4+4 | 3b | High |
| 18 | 65 | 7.5 | 4+3 | 2c | High |
| 19 | 67 | 62.7 | 4+4 | 2c | High |
| 20 | 52 | 32.5 | 4+4 | 1c | High |
Table 2.
Number of patients achieving a given PSA nadir threshold as a function of time after CyberKnifeTMtreatment
Table 3.
Acute and late lower gastrointestinal and urinary toxicity on the RTOG scale after CyberKnifeTM treatment




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download