Abstract
Longstanding, unrecognized staghorn stones remain a major cause of morbidity in the form of pain, infection, and functional impairment of the affected kidney. Squamous cell carcinoma of the upper urinary tract is associated with stone disease and chronic infection, but the association with transitional cell carcinoma (TCC) has not been proven. We report a case of a 73-year-old man presenting with right flank pain with episodes of total gross hematuria for 1 year. An abdominopelvic computed tomography scan showed decreased parenchymal enhancement and staghorn stones in the right renal pelvis and multiple tiny calyceal stones with severe hydronephrosis. The patient underwent a simple nephrectomy. Histopathologic analysis revealed staghorn stones combined with high-grade papillary TCC of the renal pelvis. The tumor was extended into the peripelvic fat and renal parenchyme (pT3NoMo).
Kim et al reported that urothelial cell carcinoma of the upper urinary tract accounts for approximately 6.0% to 7.5% of all urologic malignancies and is roughly three times more common in men than in women; the incidence rate was 0.86 to 1.21 per 100,000 persons in Korea between 1998 to 2002 [1]. Transitional cell carcinoma (TCC) makes up more than 90% of upper urinary tract tumors, followed by squamous cell carcinoma (SCC).
Longstanding staghorn stones result in functional impairment of the affected kidney. Chronic infection associated with stone disease and obstruction has been related to the development of upper urinary tract SCC. It is presumed that chronic irritation of the urothelium may result in squamous metaplasia, which can later develop into SCC [2].
On the other hand, the association between staghorn stones and TCC of the renal pelvis has rarely been documented. Preoperative diagnosis of such lesions may be difficult because of the presence of a staghorn stone and the inflammatory process.
Here we present the case of one patient who had staghorn stones and chronic renal obstruction combined with a papillary TCC of the renal pelvis.
A 73-year-old man was referred to the Sanggye Paik Hospital Emergency Center for evaluation of dizziness and general weakness. He also presented with intermittent, blunt, right flank pain and total gross hematuria lasting 1 year. He was a nonsmoker with no history of urolithiasis. He had hypertension and a previous cardiovascular accident as co-morbid conditions two years ago.
A soft mass was palpable in the right flank area by a bimanual examination. Complete blood count, urine analysis, urine culture, and routine chemistry were done. The patient's initial hemoglobin was 6.9 g/dl and his serum creatinine was 2.3 mg/dl. Urine analysis showed many red blood cell (RBC)/10-29 white blood cell (WBC) on high-power fields (HPF), and the urine culture was sterile. Urine cytology showed no abnormal cells. Plain film (KUB) radiography showed a 4×4 cm and 4×2.5 cm sized calcification and sand-like calcification on the right upper quadrant (Fig. 1).
Staghorn stones in the right renal pelvis and multiple calyceal stones with severe hydronephrosis were detected by abdominal ultrasonography, but the left kidney was in the normal range. After conservative treatment (e.g., blood transfusions and hydrations), the patient's serum creatinine level was reduced to 1.7 mg/dl. Abdominopelvic computed tomography (CT) showed staghorn stones in the right pelvis and multiple tiny calyceal stones with severe hydronephrosis. The CT scan also showed decreased renal parenchymal enhancement around the stones and diffuse cortical thinning with fluid collection (Fig. 2). No renal vein thrombi or enlarged lymph nodes were found. Therefore, we did not suspect any malignancy on these radiologic images.
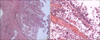
In these examinations, we concluded staghorn stones and calyceal stones were associated with the poorly functioning kidney. We performed a simple nephrectomy. In the operative field, the enlarged cystic-shaped right kidney was dark colored and easily dissected (Fig. 3). The histopathology demonstrated high-grade (WHO, 2004) papillary TCC approximately 10 cm in its greatest dimension with extension to the peripelvic fat and the full thickness of the renal parenchyma (pT3) (Fig. 4). The ureteral resection margin was involved with carcinoma. The patient refused a further operation for a ureteral stump resection. He was treated with two cycles of systemic chemotherapy with gemcitabine and carboplatin instead of cisplatin because of his lower creatinine clearance rate (42 ml/min).
Staghorn stones are primarily composed of mixtures of struvite (magnesium ammonium phosphate) and calcium carbonate apatite. These stones are typically associated with urinary tract infections with urea-splitting organisms and are referred to as infection stones. Over time, untreated staghorn stones may lead to deterioration of renal function, pyelonephritis, flank pain, end-stage renal disease, and life-threatening urosepsis may rarely be associated with urothelial carcinoma [3-7].
Raghavendran et al reported 18 patients with renal pelvis malignancies associated with a long history of staghorn stone disease (mean 8.8 years) with a non-visualized kidney on intravenous urogram [4]. A high incidence of SCC (15/18) was noted, followed by 2 TCCs and 1 adenocarcinoma (AC). The two patients with gross hematuria were diagnosed with a tumor in preoperative imaging (CT) and underwent radical nephrectomy. On the other hand, 16 patients who underwent simple nephrectomy were found to have malignancy postoperatively on histological examinations of the nephrectomized specimens. Those authors suggested that the presence of gross hematuria in these patients should raise suspicion of a renal tumor and necessitate further imaging study such as CT. They suggested a hypothesis that urothelial hyperplasia can evolve into frank TCC or it can become dysplastic due to further irritation and dedifferentiate into SCC and AC. At present, we cannot definitely say that there is a strong association between stones and TCC, but further studies are needed to prove this possible association. Although our patient presented a chief complaint of gross hematuria, we did not suspect a urothelial tumor on radiologic imaging (ultrasonography and CT) except for staghorn stones with severe hydronephrosis.
Katz et al reported that 3 cases of 500 percutaneous nephrolithotomies were revealed to have unexpected urothelial carcinoma of the renal pelvis during or after percutaneous stone removal [5]. All 3 patients had a longstanding history of stone disease and urinary infection. They all died from metastatic disease within 2-19 months after the diagnosis of urothelial cancer. In this literature, radiological investigations are not diagnostic because of reactive fibrosis and post-obstructive inflammatory changes [6]. It is common to miss an associated urothelial tumor in these patients preoperatively, so the prognosis is poor.
The prevalence of malignancy associated with a nonfunctioning kidney caused by stone disease remains unclear. Yeh et al reported that 24 of 47 (51.0%) patients who underwent nephrectomy to treat a nonfunctioning kidney caused by stone disease were revealed to have a high incidence of malignancy, such as 17 TCCs, 5 renal cell carcinomas, 1 SCC, and 1 epidermoid carcinoma [7]. Malignancy was suspected in only 7 patients in preoperative imaging studies and postoperative diagnosis of TCC was most common, although previous studies have reported a high proportion of SCC associated with nonfunctioning kidneys. Those authors suggested that surgical specimens be taken to pathology for careful evaluation during surgery in order to evaluate whether a more invasive surgery such as nephroureterectomy is necessary.
TCC of upper tract tumors may show up as papillary or sessile lesions and may be unifocal or multifocal. On histologic examination, these lesions are similar to TCC of the bladder, but the relative thinness of the muscle layer of the renal pelvis makes invasion through the muscle coat an earlier event. Progression to muscle invasion or invasion into the renal parenchyma may be more likely to occur [8]. Clinical outcomes are most highly dependent on pathologic stage and grade. Locally advanced upper tract urothelial carcinoma has a poor prognosis because of the high risk of systemic recurrence. Therefore, there is a need for effective adjuvant therapies for patients with locally advanced or metastatic disease. Adjuvant radiotherapy is of limited value in improving local disease control or survival in patients undergoing radical surgery for treatment of advanced TCC of the upper urinary tract [9]. Chemotherapy may provide some benefit. The chemotherapy regimens are the same as used for bladder cancer. After two cycles of systemic chemotherapy with gemcitabine and carboplatin during two months, we will evaluate with a short-term CT follow-up. The patient's prognosis will be poor due to the renal parenchymal invasion, positive ureteral resection margin, and lymphovascular invasion.
Our experience and other reports in the literature suggest that we should keep in mind the possibility of an underlying carcinoma of the upper urinary tract in patients with a nonfunctioning kidney and gross hematuria caused by stone disease. Before performing an operation on these patients, preoperative imaging studies should be carefully investigated to diagnose any underlying carcinoma. If any suspicion exists even after the operation, a frozen biopsy or postoperative specimen analysis should be conducted to rule out carcinoma, even if carcinoma is missed preoperatively, because early diagnosis is very important for prolongation of survival.
Figures and Tables
Fig. 1
KUB showing a staghorn stone in the right pelvis and multiple calyceal stones in a 73-year-old man.
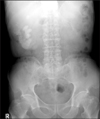
Fig. 2
Abdominopelvic CT scan showing severe hydronephrosis with diffuse cortical thinning (A) and decreased renal parenchymal enhancement around the staghorn stones in the arterial phase (B).
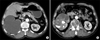
Fig. 3
Gross appearance. (A) A staghorn stone (arrow, 4×2.8×1.8 cm) is impacted in the pelvis with marked cystic dilatation of the inflamed pelvo-calyceal system; the other dilated spaces are filled with numerous small brownish stones (asterisk). (B) The pelvo-calyceal wall is thickened with a whitish granular tumor growth and mucosal papillary protrusions after formalin-fixation (arrows).

Fig. 4
Microscopic appearance. (A) Some mucosal surface shows papillary nodularity (H&E, ×10). The tumor extends to the full depth of the renal parenchyma through the pelvic wall (T3). (B) The medium power view shows a thin and delicate papillary core with attached urothelial cells with moderate differentiation (H&E, ×40).
References
1. Kim WJ, Chung JI, Hong JH, Kim CS, Jung SI, Yoon DK. Epidemiological study for urologic cancer in Korea (1998-2002). Korean J Urol. 2004. 45:1081–1088.
2. Holmäng S, Lele SM, Johansson SL. Squamous cell carcinoma of the renal pelvis and ureter: incidence, symptoms, treatment and outcome. J Urol. 2007. 178:51–56.
3. Preminger GM, Assimos DG, Lingeman JE, Nakada SY, Pearle MS, Wolf JS Jr. Chapter 1: AUA guideline on management of staghorn calculi: diagnosis and treatment recommendations. J Urol. 2005. 173:1991–2000.
4. Raghavendran M, Rastogi A, Dubey D, Chaudhary H, Kumar A, Srivastava A, et al. Stones associated renal pelvic malignancies. Indian J Cancer. 2003. 40:108–112.
5. Katz R, Gofrit ON, Golijanin D, Landau EH, Shapiro A, Pode D, et al. Urothelial cancer of the renal pelvis in percutaneous nephrolithotomy patients. Urol Int. 2005. 75:17–20.
6. Cholankeril JV, Freundlich R, Ketyer S, Spirito AL, Napolitano J. Computed tomography in urothelial tumors of renal pelvis and related filling defects. J Comput Tomogr. 1986. 10:263–272.
7. Yeh CC, Lin TH, Wu HC, Chang CH, Chen CC, Chen WC. A high association of upper urinary tract transitional cell carcinoma with nonfunctioning kidney caused by stone disease in Taiwan. Urol Int. 2007. 79:19–23.
8. Robert CF. Wein AJ, Kovoussi LR, Novick AC, Partin AW, Peters CA, editors. Urothelial tumors of the upper urinary tract. Campbell-Walsh urology. 2007. 9th ed. Philadelphia: Saunders;1643.
9. Hall MC, Womack JS, Roehrborn CG, Carmody T, Sagalowsky AI. Advanced transitional cell carcinoma of the upper urinary tract: patterns of failure, survival and impact of postoperative adjuvant radiotherapy. J Urol. 1998. 160:703–706.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download