1. Ficarra V, Cavalleri S, Novara G, Aragona M, Artibani W. Evidence from robot-assisted laparoscopic radical prostatectomy: a systematic review. Eur Urol. 2007; 51:45–55. discussion 56. PMID:
16854519.

2. Ficarra V, Novara G, Artibani W, Cestari A, Galfano A, Graefen M, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009; 55:1037–1063. PMID:
19185977.

3. Porpiglia F, Morra I, Lucci Chiarissi M, Manfredi M, Mele F, Grande S, et al. Randomised controlled trial comparing laparoscopic and robot-assisted radical prostatectomy. Eur Urol. 2013; 63:606–614. PMID:
22840353.

4. Willis DL, Gonzalgo ML, Brotzman M, Feng Z, Trock B, Su LM. Comparison of outcomes between pure laparoscopic vs robot-assisted laparoscopic radical prostatectomy: a study of comparative effectiveness based upon validated quality of life outcomes. BJU Int. 2012; 109:898–905. PMID:
21933328.

5. Eastham JA, Scardino PT, Kattan MW. Predicting an optimal outcome after radical prostatectomy: the trifecta nomogram. J Urol. 2008; 179:2207–2210. discussion 2210-1. PMID:
18423693.

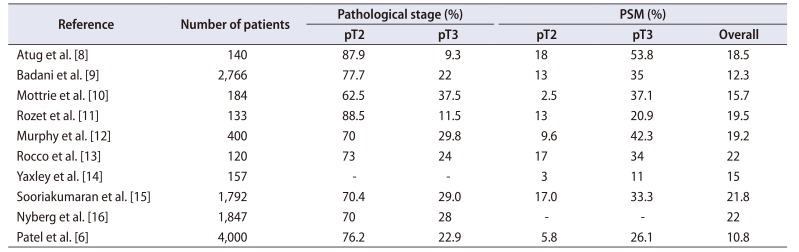
6. Patel VR, Abdul-Muhsin HM, Schatloff O, Coelho RF, Valero R, Ko YH, et al. Critical review of 'pentafecta' outcomes after robot-assisted laparoscopic prostatectomy in high-volume centres. BJU Int. 2011; 108(6 Pt 2):1007–1017. PMID:
21917104.

7. Patel VR, Sivaraman A, Coelho RF, Chauhan S, Palmer KJ, Orvieto MA, et al. Pentafecta: a new concept for reporting outcomes of robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2011; 59:702–707. PMID:
21296482.

8. Atug F, Castle EP, Srivastav SK, Burgess SV, Thomas R, Davis R. Positive surgical margins in robotic-assisted radical prostatectomy: impact of learning curve on oncologic outcomes. Eur Urol. 2006; 49:866–871. discussion 871-2. PMID:
16564614.

9. Badani KK, Kaul S, Menon M. Evolution of robotic radical prostatectomy: assessment after 2766 procedures. Cancer. 2007; 110:1951–1958. PMID:
17893904.
10. Mottrie A, Van Migem P, De Naeyer G, Schatteman P, Carpentier P, Fonteyne E. Robot-assisted laparoscopic radical prostatectomy: oncologic and functional results of 184 cases. Eur Urol. 2007; 52:746–750. PMID:
17329020.

11. Rozet F, Jaffe J, Braud G, Harmon J, Cathelineau X, Barret E, et al. A direct comparison of robotic assisted versus pure laparoscopic radical prostatectomy: a single institution experience. J Urol. 2007; 178:478–482. PMID:
17561160.

12. Murphy DG, Kerger M, Crowe H, Peters JS, Costello AJ. Operative details and oncological and functional outcome of robotic-assisted laparoscopic radical prostatectomy: 400 cases with a minimum of 12 months follow-up. Eur Urol. 2009; 55:1358–1366. PMID:
19147274.

13. Rocco B, Matei DV, Melegari S, Ospina JC, Mazzoleni F, Errico G, et al. Robotic vs open prostatectomy in a laparoscopically naive centre: a matched-pair analysis. BJU Int. 2009; 104:991–995. PMID:
19426191.

14. Yaxley JW, Coughlin GD, Chambers SK, Occhipinti S, Samaratunga H, Zajdlewicz L, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet. 2016; 388:1057–1066. PMID:
27474375.

15. Sooriakumaran P, Pini G, Nyberg T, Derogar M, Carlsson S, Stranne J, et al. Erectile function and oncologic outcomes following open retropubic and robot-assisted radical prostatectomy: results from the laparoscopic prostatectomy robot open trial. Eur Urol. 2018; 73:618–627. PMID:
28882327.

16. Nyberg M, Hugosson J, Wiklund P, Sjoberg D, Wilderäng U, Carlsson SV, et al. LAPPRO group. Functional and oncologic outcomes between open and robotic radical prostatectomy at 24-month follow-up in the Swedish LAPPRO trial. Eur Urol Oncol. 2018; 1:353–360. PMID:
31158073.

17. Kang SG, Schatloff O, Haidar AM, Samavedi S, Palmer KJ, Cheon J, et al. Overall rate, location, and predictive factors for positive surgical margins after robot-assisted laparoscopic radical prostatectomy for high-risk prostate cancer. Asian J Androl. 2016; 18:123–128. PMID:
25966623.

18. Coelho RF, Chauhan S, Orvieto MA, Palmer KJ, Rocco B, Patel VR. Predictive factors for positive surgical margins and their locations after robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2010; 57:1022–1029. PMID:
20163911.

19. Patel VR, Sandri M, Grasso AAC, De Lorenzis E, Palmisano F, Albo G, et al. A novel tool for predicting extracapsular extension during graded partial nerve sparing in radical prostatectomy. BJU Int. 2018; 121:373–382. PMID:
28941058.

20. Schatloff O, Chauhan S, Kameh D, Valero R, Ko YH, Sivaraman A, et al. Cavernosal nerve preservation during robot-assisted radical prostatectomy is a graded rather than an all-or-none phenomenon: objective demonstration by assessment of residual nerve tissue on surgical specimens. Urology. 2012; 79:596–600. PMID:
22386406.

21. Coelho RF, Chauhan S, Palmer KJ, Rocco B, Patel MB, Patel VR. Robotic-assisted radical prostatectomy: a review of current outcomes. BJU Int. 2009; 104:1428–1435. PMID:
19804427.

22. Rocco B, Gregori A, Stener S, Santoro L, Bozzola A, Galli S, et al. Posterior reconstruction of the rhabdosphincter allows a rapid recovery of continence after transperitoneal videolaparoscopic radical prostatectomy. Eur Urol. 2007; 51:996–1003. PMID:
17079070.

23. Joseph JV, Rosenbaum R, Madeb R, Erturk E, Patel HR. Robotic extraperitoneal radical prostatectomy: an alternative approach. J Urol. 2006; 175(3 Pt 1):945–950. PMID:
16469589.

24. Zorn KC, Gofrit ON, Orvieto MA, Mikhail AA, Zagaja GP, Shalhav AL. Robotic-assisted laparoscopic prostatectomy: functional and pathologic outcomes with interfascial nerve preservation. Eur Urol. 2007; 51:755–762. PMID:
17084520.

25. Tewari A, Jhaveri J, Rao S, Yadav R, Bartsch G, Te A, et al. Total reconstruction of the vesico-urethral junction. BJU Int. 2008; 101:871–877. PMID:
18321319.

26. Shikanov SA, Zorn KC, Zagaja GP, Shalhav AL. Trifecta outcomes after robotic-assisted laparoscopic prostatectomy. Urology. 2009; 74:619–623. PMID:
19592075.

27. Patel VR, Coelho RF, Chauhan S, Orvieto MA, Palmer KJ, Rocco B, et al. Continence, potency and oncological outcomes after robotic-assisted radical prostatectomy: early trifecta results of a high-volume surgeon. BJU Int. 2010; 106:696–702. PMID:
20707793.

28. Haglind E, Carlsson S, Stranne J, Wallerstedt A, Wilderäng U, Thorsteinsdottir T, et al. LAPPRO steering committee. Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: a prospective, controlled, nonrandomised trial. Eur Urol. 2015; 68:216–225. PMID:
25770484.

29. Coughlin GD, Yaxley JW, Chambers SK, Occhipinti S, Samaratunga H, Zajdlewicz L, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol. 2018; 19:1051–1060. PMID:
30017351.

30. Ficarra V, Novara G, Rosen RC, Artibani W, Carroll PR, Costello A, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. 2012; 62:405–417. PMID:
22749852.

31. Link BA, Nelson R, Josephson DY, Yoshida JS, Crocitto LE, Kawachi MH, et al. The impact of prostate gland weight in robot assisted laparoscopic radical prostatectomy. J Urol. 2008; 180:928–932. PMID:
18635217.

32. Novara G, Ficarra V, D'elia C, Secco S, Cioffi A, Cavalleri S, et al. Evaluating urinary continence and preoperative predictors of urinary continence after robot assisted laparoscopic radical prostatectomy. J Urol. 2010; 184:1028–1033. PMID:
20643426.

33. Shikanov S, Desai V, Razmaria A, Zagaja GP, Shalhav AL. Robotic radical prostatectomy for elderly patients: probability of achieving continence and potency 1 year after surgery. J Urol. 2010; 183:1803–1807. PMID:
20299041.

34. van der Poel HG, de Blok W, Joshi N, van Muilekom E. Preservation of lateral prostatic fascia is associated with urine continence after robotic-assisted prostatectomy. Eur Urol. 2009; 55:892–900. PMID:
19171418.

35. Lin VC, Coughlin G, Savamedi S, Palmer KJ, Coelho RF, Patel VR. Modified transverse plication for bladder neck reconstruction during robotic-assisted laparoscopic prostatectomy. BJU Int. 2009; 104:878–881. PMID:
19706036.

36. Walsh PC. Anatomic radical prostatectomy: evolution of the surgical technique. J Urol. 1998; 160(6 Pt 2):2418–2424. PMID:
9817395.
37. Patel VR, Coelho RF, Palmer KJ, Rocco B. Periurethral suspension stitch during robot-assisted laparoscopic radical prostatectomy: description of the technique and continence outcomes. Eur Urol. 2009; 56:472–478. PMID:
19560260.

38. Vis AN, van der Poel HG, Ruiter AEC, Hu JC, Tewari AK, Rocco B, et al. Posterior, anterior, and periurethral surgical reconstruction of urinary continence mechanisms in robotassisted radical prostatectomy: a description and video compilation of commonly performed surgical techniques. Eur Urol. 2019; 76:814–822. PMID:
30514568.

39. Gautam G, Rocco B, Patel VR, Zorn KC. Posterior rhabdosphincter reconstruction during robot-assisted radical prostatectomy: critical analysis of techniques and outcomes. Urology. 2010; 76:734–741. PMID:
20579697.

40. Joshi N, de Blok W, van Muilekom E, van der Poel H. Impact of posterior musculofascial reconstruction on early continence after robot-assisted laparoscopic radical prostatectomy: results of a prospective parallel group trial. Eur Urol. 2010; 58:84–89. PMID:
20362386.

41. Freire MP, Weinberg AC, Lei Y, Soukup JR, Lipsitz SR, Prasad SM, et al. Anatomic bladder neck preservation during robotic-assisted laparoscopic radical prostatectomy: description of technique and outcomes. Eur Urol. 2009; 56:972–980. PMID:
19781848.

42. Coelho RF, Chauhan S, Orvieto MA, Sivaraman A, Palmer KJ, Coughlin G, et al. Influence of modified posterior reconstruction of the rhabdosphincter on early recovery of continence and anastomotic leakage rates after robot-assisted radical prostatectomy. Eur Urol. 2011; 59:72–80. PMID:
20801579.

43. de Carvalho PA, Barbosa JABA, Guglielmetti GB, Cordeiro MD, Rocco B, Nahas WC, et al. Retrograde release of the neurovascular bundle with preservation of dorsal venous complex during robot-assisted radical prostatectomy: optimizing functional outcomes. Eur Urol. 2018; 7. 21. DOI:
10.1016/j.eururo.2018.07.003. [Epub].

44. Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. 1982. J Urol. 2002; 167(2 Pt 2):1005–1010. PMID:
11905870.
45. Novara G, Ficarra V, D'Elia C, Secco S, De Gobbi A, Cavalleri S, et al. Preoperative criteria to select patients for bilateral nerve-sparing robotic-assisted radical prostatectomy. J Sex Med. 2010; 7(2 Pt 1):839–845. PMID:
19912486.

46. Briganti A, Gallina A, Suardi N, Capitanio U, Tutolo M, Bianchi M, et al. Predicting erectile function recovery after bilateral nerve sparing radical prostatectomy: a proposal of a novel preoperative risk stratification. J Sex Med. 2010; 7:2521–2531. PMID:
20487236.

47. Marien T, Sankin A, Lepor H. Factors predicting preservation of erectile function in men undergoing open radical retropubic prostatectomy. J Urol. 2009; 181:1817–1822. PMID:
19233413.

48. Moskovic DJ, Alphs H, Nelson CJ, Rabbani F, Eastham J, Touijer K, et al. Subjective characterization of nerve sparing predicts recovery of erectile function after radical prostatectomy: defining the utility of a nerve sparing grading system. J Sex Med. 2011; 8:255–260. PMID:
20727065.

49. Kang SG, Schatloff O, Haidar AM, Samavedi S, Palmer KJ, Cheon J, et al. Does surgeon subjective nerve sparing score predict recovery time of erectile function following robot-assisted radical prostatectomy? J Sex Med. 2015; 12:1490–1496. PMID:
25689342.

50. Menon M, Shrivastava A, Kaul S, Badani KK, Fumo M, Bhandari M, et al. Vattikuti Institute prostatectomy: contemporary technique and analysis of results. Eur Urol. 2007; 51:648–657. discussion 657-8. PMID:
17097214.

51. Finley DS, Rodriguez E Jr, Skarecky DW, Ahlering TE. Quantitative and qualitative analysis of the recovery of potency after radical prostatectomy: effect of unilateral vs bilateral nerve sparing. BJU Int. 2009; 104:1484–1489. PMID:
19388985.

52. Coughlin G, Dangle PP, Palmer KJ, Samevedi S, Patel VR. Athermal early retrograde release of the neurovascular bundle during nerve-sparing robotic-assisted laparoscopic radical prostatectomy. J Robot Surg. 2009; 3:13–17. PMID:
27628447.

53. Lei Y, Alemozaffar M, Williams SB, Hevelone N, Lipsitz SR, Plaster BA, et al. Athermal division and selective suture ligation of the dorsal vein complex during robot-assisted laparoscopic radical prostatectomy: description of technique and outcomes. Eur Urol. 2011; 59:235–243. PMID:
20863611.

54. Takenaka A, Leung RA, Fujisawa M, Tewari AK. Anatomy of autonomic nerve component in the male pelvis: the new concept from a perspective for robotic nerve sparing radical prostatectomy. World J Urol. 2006; 24:136–143. PMID:
16758247.

55. Tavukçu HH, Aytac O, Atug F. Nerve-sparing techniques and results in robot-assisted radical prostatectomy. Investig Clin Urol. 2016; 57(Suppl 2):S172–S184.

56. Asimakopoulos AD, Annino F, D'Orazio A, Pereira CF, Mugnier C, Hoepffner JL, et al. Complete periprostatic anatomy preservation during robot-assisted laparoscopic radical prostatectomy (RALP): the new pubovesical complex-sparing technique. Eur Urol. 2010; 58:407–417. PMID:
20825759.

57. Menon M, Shrivastava A, Bhandari M, Satyanarayana R, Siva S, Agarwal PK. Vattikuti Institute prostatectomy: technical modifications in 2009. Eur Urol. 2009; 56:89–96. PMID:
19403236.

58. Curto F, Benijts J, Pansadoro A, Barmoshe S, Hoepffner JL, Mugnier C, et al. Nerve sparing laparoscopic radical prostatectomy: our technique. Eur Urol. 2006; 49:344–352. PMID:
16413102.

59. Ko YH, Coelho RF, Sivaraman A, Schatloff O, Chauhan S, Abdul-Muhsin HM, et al. Retrograde versus antegrade nerve sparing during robot-assisted radical prostatectomy: which is better for achieving early functional recovery? Eur Urol. 2013; 63:169–177. PMID:
23092543.

60. Costello AJ, Brooks M, Cole OJ. Anatomical studies of the neurovascular bundle and cavernosal nerves. BJU Int. 2004; 94:1071–1076. PMID:
15541130.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download