Abstract
Purpose
Classic angiomyolipoma (AML) is common benign kidney tumor. However, some studies have claimed that epithelioid angiomyolipoma (EAML) has malignant potential. We compared the patient characteristics and prognosis of EAML and classic AML to demonstrate predicting factors and poorer prognosis of EAML.
Materials and Methods
The medical records of 231 patients who were diagnosed with EAML (n=27, 11.7%) or classic AML (n=204, 88.3%), were reviewed. All patients underwent computed tomography (CT) scans before operation or needle biopsy. We assessed the age, sex, tumor size, body mass index, comorbidities, and Hounsfield unit (HU) according to each CT phase. We defined the unfavorable group as patients with recurrence, metastasis and death due to tumor progression. Logistic regression analysis was used to predict EAML.
Results
EAML patients were younger (41.2 years vs. 49.1 years, p=0.001), predominantly male (55.6% vs. 28.4%, p=0.005), and had a larger tumor (7.5 cm vs. 4.2 cm, p<0.001). The median pre-contrast HU was not significantly different between EAML and classic AML (29.9±23.7 vs. 14.7±41.0, p=0.071). In multivariable analysis, younger age (odds ratio [OR], 0.96; p=0.032), male sex (OR, 3.33; p=0.013), and tumor larger than 4 cm (OR, 3.8; p=0.009) were significant predictive factors. Five patients (18.5%) had unfavorable outcomes, two patients had lymph node metastasis, and three patients had lung metastasis.
Angiomyolipomas (AMLs) are predominantly found in the kidneys and less frequently found in extra-renal sites, such as the liver and retroperitoneum [1]. Renal AML is one of the most common renal benign tumors, which is a histologically complex mesenchymal tumor composed of fat cells, spindled smooth muscle cells, and dysmorphic blood vessels [2]. Studies have shown that renal AML accounts for approximately 1% of all renal tumors. Both sexes are equally affected by renal AML and the mean age at diagnosis is 38 years [23]. There are two types of renal AML, classic AML and epithelioid AML (EAML), classified according to the 2004 World Health Organization (WHO) classification of tumors [4]. Classic renal AML is a benign mesenchymal tumor [25]. On the other hand, EAML is a potentially malignant mesenchymal tumor of the kidneys, part of the family of tumors with perivascular epithelioid cell differentiation [1]. EAML is partially or entirely composed of atypical large epithelioid cells with abundant cytoplasm, vesicular nuclei, and prominent nucleoli [6]. It also exhibits a unique immuno-histochemical profile. Typically, EAML stains positive for melanocytic markers (Human Melanoma Black [HMB]-45, HMB-50, Mart-1/Melan-A, and microphthalmia-associated transcription factor) as well as smooth muscle markers (smooth muscle actin, and muscle-specific actin) due to the presence of epithelioid cells [3]. EAMLs and their relationship with classic AML were first reported by Martignoni et al. [7] and Mai et al. [8], who demonstrated the poorer clinical outcomes of EAML.
Many studies of EAML have revealed its malignant potential; however, most of these studies include only a few case reports, and the follow-up periods are not long enough. Moreover, AML is not truly malignant according to the WHO [9]. Therefore, knowledge of the characteristics and prognosis of EAML is insufficient. The primary endpoint of this study was to identify the differences in clinical characteristics and long-term prognosis between EAML and classic AML to clarify the malignant potential and predicting factors of EAML. In addition, since there are no clinical or specific radiological criteria that characterize EAML, we attempted to determine the different clinical and radiographic features of EAML to differentiate it from classic AML before pathological diagnosis.
After receiving approval from the Institutional Review Board of Asan Medical Center (approval number: 2014-0498), the medical records of 231 patients diagnosed with classic AML or EAML in Asan Medical Center were reviewed from 2000 to 2015. The patients were pathologically diagnosed as classic AML or EAML by nephrectomy or needle biopsy. The patients were recommended to perform nephrectomy or needle biopsy when there was increase in tumor size on follow-up radiographic examination, such as multiphasic computed tomography (CT) or ultrasonography, even though the tumor was considered as a benign classic AML. Additionally, when the formal reading of radiographic examination by radiologist suggested a possibility of malignancy, nephrectomy or needle biopsy was performed. Fig. 1 shows the pathological differences between the two types of AML. Classic AML was defined pathologically as a tumor that is composed of varying proportions of smooth muscle cells, fat cells and vascular cells. EAML was defined as a tumor that is predominantly composed of epithelioid cells. The exact criteria of the amount or ratio of epithelioid cells for diagnosing EAML pathologically were not defined. In this study, EAML was diagnosed when the pathologic specimens contained a high proportion of epithelioid cells and characteristically stained positive for HMB-45.
Multiphasic CT was performed for all of the enrolled patients. The Hounsfield unit (HU) of the tumors on contrast-enhanced CT images, age, sex, size of the tumor, body mass index, and comorbidities were analyzed to determine factors that could contribute to the poor prognosis of EAML. The unfavorable group was defined as patients with recurrence, metastasis and death due to tumor progression. The variables were compared by univariate analysis and multiple logistic regression analysis to identify factors for diagnosing EAML.
Of the 231 patients, 27 EAML patients (11.7%) were identified. Table 1 shows the patient characteristics of patients with AML and EAML. The median age of the EAML group was younger compared with that of the classic AML group (41.2±12.3 vs. 49.1±11.6, p=0.001). The median size of the tumor was 7.5±4.7 cm in the EAML group and 4.2±4.4 cm in the classical AML (p<0.001). In addition, 5.4% of classic AML patients and 7.4% of EAML patients had diabetes mellitus (p=0.978). Classic AML patients had higher incidence of hypertension (20.1% vs. 0.0%, p=0.024). The median precontrast HU was 14.7±41.0 in the classic AML group and 29.9±23.7 in the EAML group (p=0.071). The median HU in the arterial phase was 79.9±55.0 and 76.6±40.5 in the classic AML group and EAML group, respectively (p=0.769). The median difference in the HU between the arterial phase and pre-contrast phase was 65.7±39.7 in the classic AML group and 46.6±34.6 in the EAML group (p=0.022). The median difference in the HU between the arterial phase and venous phase was −1.1±30.0 and 4.1±22.0 in the classic AML group and EAML group, respectively (p=0.426). The median follow-up duration was 30.5±33.7 months in the classic AML group and 46.3±43.2 months in the EAML group (p=0.029).
Table 2 shows the univariate and multivariate analysis of each characteristic. The difference in the HU between the arterial phase and pre-contrast phase was statistically significant in univariate analysis (p=0.021) but not in multivariate analysis. The difference in the HU between the arterial phase and venous phase was not statistically significant. Age, sex, and the size of the tumor mass on CT images were statistically significant in multivariate analysis. The odds ratio (OR) was decreased by 4.0% for every age increase of 1 year (OR, 0.96; 95% confidence interval [CI], 0.92−0.99). Males were 3.33 times more likely than females to be diagnosed with EAML (OR, 3.33; 95% CI, 1.30−7.56). If the tumor mass on CT images was larger than 4 cm, there was a 3.8 times higher chance of being diagnosed with EAML (OR, 3.80; 95% CI, 1.62−11.08).
Five EAML patients (18.5%) showed unfavorable outcomes, defined as metastasis, recurrence, and death due to tumor progression. Two patients in the unfavorable group had lymph node metastasis in the para-aortic or aortic area with no metastasis in other sites. After radical nephrectomy and LN dissection, metastasis or local recurrence did not occur in these patients. Three of the five patients had lung metastasis. In the classic AML group, none of the patients had metastasis, and none of them expired due to tumor progression.
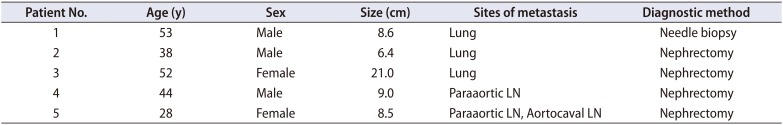
EAML is a rare disease characterized by the predominance of epithelioid cells with positive staining for melanocytic markers, such as HBM-45 [10]. According to previous studies, many patients with EAML have poor prognosis [691112]. Several case reports have shown that EAML has malignant potential and can result in mortality [13]. Distant metastasis was found to occur 1.5−9 years postoperatively, and most patients expired within 1 year of metastasis [121415]. Brimo et al. [5] reported a malignancy rate of 26%. Most case reports on EAML indicated that it is radiologically and histologically similar to renal cell carcinoma [16]. In addition, it may exhibit clinically aggressive behavior, such as metastasis or local recurrence, mimicking renal cell carcinoma. Nese et al. [13] reported that among a sample of 41 EAML patients, the rate of recurrence and metastasis was 17% and 49%, respectively. Sites of metastasis include the liver, lymph nodes, lungs, bones, and other rare sites [13]. Folpe and Kwiatkowski [17] have observed the aggressive behavior of EAML especially for tumors larger than 5 cm with an infiltrative growth pattern, high nuclear grade, and necrosis. Our study revealed a marked difference of in the prognosis between classic AML and EAML. The results are in contrast with those of a study by Aydin et al. [2], in which all cases of EAML (n=15) showed a benign course with a median follow-up of 5.1 years. Of the 27 EAML patients in our study, five of them had unfavorable outcomes. The first patient was diagnosed as having lymphoma or atypical renal cell carcinoma with peri-renal mass and para-aortic lymph node metastasis by CT. His chest CT scan showed multiple metastatic nodules in both lungs at the time of diagnosis. He also had bone metastasis in the left ileum, which was identified in positron emission tomography CT. EAML was diagnosed with the needle biopsy of the perirenal mass. He was hospitalized in the oncology department for palliative care. He refused to receive chemotherapy and expired a month later. The second patient underwent partial nephrectomy and was pathologically diagnosed with EAML. Lung metastasis was found on the followup CT scan 3 years after the surgery, and the patient expired 1 year later. The patient did not receive additional therapy due to the patient's refusal. The third patient was diagnosed with EAML after right radical nephrectomy. Chest posteroanterior (PA) view showed a left upper lung nodule 5 years after the surgery, which demonstrated slow growth when compared with that in a prior chest PA. Lung biopsy was performed, which indicated metastatic EAML. This patient expired after 2 years. There was no information on whether this patient received adjuvant chemotherapy. The other two patients in the unfavorable group had lymph node metastasis at the time of diagnosis. They underwent radical nephrectomy with lymph node dissection and tumor did not recur during the follow-up period. Table 3 shows the patient and tumor characteristics of unfavorable group. None of the 204 patients diagnosed with classic AML had metastasis or expired due to classic AML.
Despite the advancements in diagnostic techniques and increased knowledge about EAML, the prognostic factors that contribute to the malignancy potential of EAML remain unknown [13]. We found that male and younger patients had higher chance of having EAML, and the size of the tumor was an additional indicative factor for diagnosing EAML. According to the literature, most renal AMLs are small; however, they may be larger than 30 cm in diameter, and EAML is usually larger compared with classic AML [2]. Patients with tumors measuring up to 4 cm in diameter had a 3.33 times higher chance of being diagnosed with EAML.
Tumors, which are predominantly composed of smooth muscle cells or epithelioid cells, such as EAML can radiologically mimic renal cell carcinoma in the kidneys [2]. The radiographic diagnosis of EAML is difficult because abnormal blood vessels and mature fat cells are not apparent compared with those of classic AML [16]. Liu et al. [18] showed that EAML presents as a large soft tissue lesion with high density compared with normal renal parenchyma on CT images. A characteristic enhancement pattern of EAML is rapid wash-in and slow wash-out. Zhu et al. [19] reported several cases of EAML, and the radiographic characteristics were inconsistent with those of previous studies. According to the report, EAML has a wide range of imaging characteristics, some of which overlap with those of other renal tumors; thus, it is difficult to differentiate between renal cell carcinoma and fat-poor classic AML without immunohistochemistry. Most of EAMLs were diagnosed as renal cell carcinoma based on CT scans in our center. In our study, the pre-contrast HU of the tumor mass appeared to be lower for EAML but was not statistically significant. The difference in the HU between the arterial phase and pre-contrast phase was significantly lower in the EAML group in univariate analysis but not in multivariate analysis.
As EAML does not have distinctive imaging characteristics, surgical removal and needle biopsy are methods of diagnosing EAML. Special stains may be used to help diagnose EAML, such as melanocytic markers and smooth muscle markers. The percentage of the epithelioid component for classifying a tumor as EAML has not been established. Some studies have used a cut-off of 10% epithelioid cells in a given tumor to classify it as EAML [4]. Based on more than 100 case reports in four largest series, all tumors developing metastasis had ≥80% epithelioid histology [5]. In our study, a specific cut-off percentage of epithelioid cells was not used. EAML was diagnosed when epithelioid cells were predominant with positive staining for HBM-45.
Thus far, surgery is the treatment choice for EAML. However, surgery might be insufficient in some cases, such as those with local recurrence or distant metastasis. Cibas et al. [20] and Park et al. [21] used doxorubicin, cyclophosphamide, and cisplatin for EAML, and they found that these agents were effective. Benson et al. [22] recommended mammalian target of rapamycin inhibitors as a first-line therapy for patients with perivascular epithelioid cell neoplasms. However, long-term observation is needed to confirm their effects. In our study, metastatic EAML patients did not receive chemotherapy due to the patients' refusal and rapid progression causing death. Therefore, long-term follow-up and prospective studies of metastatic EAML patients who received chemotherapy with or without surgery are needed to establish an optimal therapy for EAML patients.
This study had some limitations. This was a single center, retrospective study; thus, selection bias could not be avoided. In addition, the follow-up period for classic AML had relatively shorter than that of EAML. We could not identify significant factors in radiologic examination. A larger scale study may identify diagnostic tools for radiologic or laboratory examination.
In conclusion, EAML has poorer prognosis compared with classic AML. Younger age, male sex, and larger tumor mass may increase the possibility of diagnosing of EAML. The lower HU of the mass on the pre-contrast CT scan showed a tendency to be diagnosed as EAML but was not statistically significant. EAML has malignant potential and requires careful follow-up. Because there is no established radiologic diagnostic tool for EAML, performing a needle biopsy should be considered for young and male patients with a relatively large AML (a diameter more than 4 cm) to rule out EAML.
ACKNOWLEDGMENTS
This study was supported by a grant from Asan Institute for Life Sciences, Asan Medical Center (grant number: W17-686) and Korea Health Technology R&D Project, Ministry of Health & Welfare (grant number: H16C2193).
References
1. Neumann HP, Schwarzkopf G, Henske EP. Renal angiomyolipomas, cysts, and cancer in tuberous sclerosis complex. Semin Pediatr Neurol. 1998; 5:269–275. PMID: 9874854.

2. Aydin H, Magi-Galluzzi C, Lane BR, Sercia L, Lopez JI, Rini BI, et al. Renal angiomyolipoma: clinicopathologic study of 194 cases with emphasis on the epithelioid histology and tuberous sclerosis association. Am J Surg Pathol. 2009; 33:289–297. PMID: 18852677.
3. Armah HB, Parwani AV. Perivascular epithelioid cell tumor. Arch Pathol Lab Med. 2009; 133:648–654. PMID: 19391667.

4. He W, Cheville JC, Sadow PM, Gopalan A, Fine SW, Al-Ahmadie HA, et al. Epithelioid angiomyolipoma of the kidney: pathological features and clinical outcome in a series of consecutively resected tumors. Mod Pathol. 2013; 26:1355–1364. PMID: 23599151.

5. Brimo F, Robinson B, Guo C, Zhou M, Latour M, Epstein JI. Renal epithelioid angiomyolipoma with atypia: a series of 40 cases with emphasis on clinicopathologic prognostic indicators of malignancy. Am J Surg Pathol. 2010; 34:715–722. PMID: 20410812.

6. Varma S, Gupta S, Talwar J, Forte F, Dhar M. Renal epithelioid angiomyolipoma: a malignant disease. J Nephrol. 2011; 24:18–22. PMID: 20349413.

7. Martignoni G, Pea M, Bonetti F, Brnelli M, Eble JN. Oncocytoma-like angiomyolipoma. A clinicopathologic and immunohistochemical study of 2 cases. Arch Pathol Lab Med. 2002; 126:610–612. PMID: 11958671.
8. Mai KT, Perkins DG, Collins JP. Epithelioid cell variant of renal angiomyolipoma. Histopathology. 1996; 28:277–280. PMID: 8729052.

9. Lei JH, Liu LR, Wei Q, Song TR, Yang L, Yuan HC, et al. A four-year follow-up study of renal epithelioid angiomyolipoma: a multi-center experience and literature review. Sci Rep. 2015; 5:10030. PMID: 25939249.

10. Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006; 49:798–805. PMID: 16442207.

11. Faraji H, Nguyen BN, Mai KT. Renal epithelioid angiomyolipoma: a study of six cases and a meta-analytic study. Development of criteria for screening the entity with prognostic significance. Histopathology. 2009; 55:525–534. PMID: 19912358.

12. Lavrut PM, Paparel P, Decaussin-Petrucci M. Epithelioid angiomyolipoma of the kidney: about one case and malignant features evaluation. Ann Pathol. 2017; 37:182–187. PMID: 28318774.
13. Nese N, Martignoni G, Fletcher CD, Gupta R, Pan CC, Kim H, et al. Pure epithelioid PEComas (so-called epithelioid angiomyolipoma) of the kidney: a clinicopathologic study of 41 cases: detailed assessment of morphology and risk stratification. Am J Surg Pathol. 2011; 35:161–176. PMID: 21263237.
14. Huang KH, Huang CY, Chung SD, Pu YS, Shun CT, Chen J. Malignant epithelioid angiomyolipoma of the kidney. J Formos Med Assoc. 2007; 106(2 Suppl):S51–S54. PMID: 17493897.

15. Yamamoto T, Ito K, Suzuki K, Yamanaka H, Ebihara K, Sasaki A. Rapidly progressive malignant epithelioid angiomyolipoma of the kidney. J Urol. 2002; 168:190–191. PMID: 12050523.

16. Adanur S, Keskin E, Ziypak T, Koc E, Demirci E, Yapanoglu T, et al. Renal epithelioid angiomyolipoma mimicking urothelial carcinoma of the upper urinary tract. Arch Ital Urol Androl. 2014; 86:235–236. PMID: 25308597.

17. Folpe AL, Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol. 2010; 41:1–15. PMID: 19604538.

18. Liu Y, Qu F, Cheng R, Ye Z. CT-imaging features of renal epithelioid angiomyolipoma. World J Surg Oncol. 2015; 13:280. PMID: 26391670.

19. Zhu J, Li H, Ding , L , Cheng H. Imaging appearance of renal epithelioid angiomyolipoma: a case report and literature review. Medicine (Baltimore). 2018; 97:e9563. PMID: 29505538.
20. Cibas ES, Goss GA, Kulke MH, Demetri GD, Fletcher CD. Malignant epithelioid angiomyolipoma (‘sarcoma ex angiomyolipoma’) of the kidney: a case report and review of the literature. Am J Surg Pathol. 2001; 25:121–126. PMID: 11145246.
21. Park HK, Zhang S, Wong MK, Kim HL. Clinical presentation of epithelioid angiomyolipoma. Int J Urol. 2007; 14:21–25. PMID: 17199855.

22. Benson C, Vitfell-Rasmussen J, Maruzzo M, Fisher C, Tunariu N, Mitchell S, et al. A retrospective study of patients with malignant PEComa receiving treatment with sirolimus or temsirolimus: the royal marsden hospital experience. Anticancer Res. 2014; 34:3663–3668. PMID: 24982384.
Fig. 1
Histology of classic angiomyolipoma (AML) and epithelioid angiomyolipoma (EAML). (A) Classic AML composed of different proportions of smooth muscle cells, adipose tissue, and blood vessels (H&E, ×100). (B) EAML composed of a large number of hyperplastic epithelioid cells (H&E, ×100). (C) with positive staining for HBM-45 (Human Melanoma Black-45, ×100).
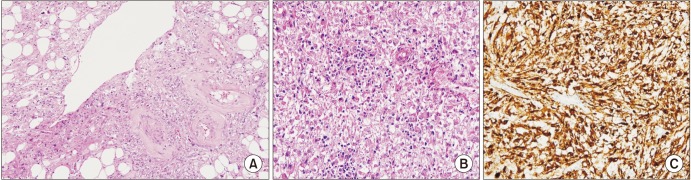
Table 1
Demographic and pathologic characteristics
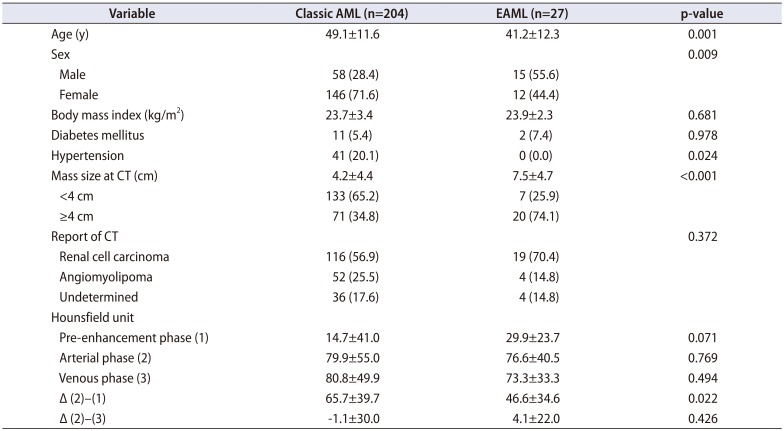
Table 2
Logistic regression to predict factors associated with epithelioid angiomyolipoma
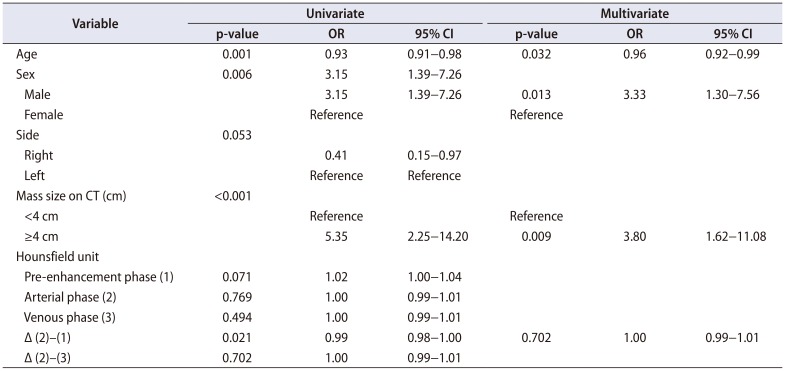
Table 3
Patient and tumor characteristics of unfavorable group




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download