Abstract
Generally accepted guidelines are not yet available on the management of underactive bladder (UAB). Although the natural history of UAB is still not fully understood, observation may be an acceptable management option in patients with tolerable lower urinary tract symptoms and little risk of upper urinary tract damage. If needed, scheduled and double voiding may be recommended as an effective and safe add-on therapy. Parasympathomimetics have been widely used for the management of UAB, but the evidence does not support clinical benefit. The efficacy of alpha-blockers has also not yet been clearly demonstrated. However, selective alpha-blockers may help to enhance voiding efficiency and to decrease possible upper tract damage. Sacral neuromodulation is a surgical option for nonobstructive UAB approved by the Food and Drug Administration. However, the response rate of test stimulation is not high and the efficacy of permanent implants does not always coincide with that of test stimulation. Although surgery to reduce outlet resistance may be a viable option in UAB with presumed obstruction, surgery seems to have little role in those without obstruction. Latissimus dorsi detrusor myoplasty has shown promising results in restoring voluntary voiding in selected patients. The procedure requires a multidisciplinary team approach of urologists and plastic reconstructive experts. In summary, current treatments of UAB remain unsatisfactory. The multifactorial nature of UAB pathogenesis complicates the appropriate management for each patient. Future research to establish a more clinically relevant definition of UAB will be required to open new era of UAB management.
Underactive bladder (UAB) has not yet been formally defined but has been suggested to be a clinical manifestation of detrusor underactivity characterized by a weak, poorly sustained urinary stream and high postvoid residual urine volume (PVRU) [1]. Although voiding lower urinary tract symptoms (LUTS) constitute the major discomfort of UAB, storage LUTS such as frequency, nocturia, and incontinence are often present. Many authors have used their own working definitions for detrusor underactivity or UAB while conducting basic and clinical research. In this review, for simplicity of description, the term UAB will be used to represent each of the relevant working definitions of detrusor underactivity or UAB in the references.
UAB is basically an emptying failure resulting from insufficient generation of adequate intravesical pressure to void. The bladder performs the main role in generating intravesical pressure and is generally regarded as the target organ in the management of UAB. However, it should be noted that many other pathologies in the voiding reflex arc or bladder outlet can contribute to the development of UAB, which may be other potential targets for management [2].
Regardless of the cause of UAB in each patient, the management strategy should focus on improving the patient's quality of life and delaying the development of possible complications, such as recurrent urinary tract infections, bladder stones, and upper urinary tract damage leading to chronic renal failure [3]. Although the natural history of UAB is still not fully understood, observation may be an acceptable management option for patients with tolerable LUTS and little risk of upper urinary tract damage. The low-pressure bladder in some UAB patients may protect the upper urinary tract from self-inflicted pressure damage. In a long-term follow-up study of untreated UAB (69 male patients), 84% remained untreated after a mean follow-up of 13.6 years [4]. No significant clinical changes were reported over the follow-up periods.
So far, no generally accepted guidelines are available on the management of UAB. As an effective and safe add-on therapy, scheduled voiding and double voiding may be recommended to avoid overdistension and decrease PVRU [3]. However, manual external bladder compression (Credé maneuver) or abdominal straining (Valsalva maneuver) are not recommended owing to the risk of developing high intravesical pressure and upper tract damage. Moreover, these maneuvers can elicit a reflex sphincter contraction with the result of increasing outlet resistance [56].
If the patient's overall clinical condition indicates risk of progression or worsening of intolerable LUTS, therapeutic intervention including catheterization or pharmacological or surgical management options should be considered. Many management options have been developed to enhance bladder emptying in each step of the voiding process. Traditional approaches focus on the bladder, the final target organ in the process, to increase detrusor contractility by stimulating neurotransmitter secretion or adding more detrusor power. Modulation of the neural circuit involving the voiding reflex and central nervous system (CNS) facilitates a coordinated detrusor contraction. Also, decreasing outlet resistance by medication or surgery can work in a select group of UAB patients.
The first strategy for UAB pharmacotherapy is to increase detrusor contractility. Since detrusor contraction is mediated by parasympathetic innervation, enhancement of parasympathetic activity would be expected to increase detrusor contraction. Parasympathomimetics are substances to stimulate the parasympathetic nervous system in the body. Parasympathomimetics can act either directly by stimulating the muscarinic receptors or indirectly by inhibiting cholinesterase and thus promoting acetylcholine release [7].
Bethanechol chloride is a parasympathomimetic choline carbamate that selectively stimulates muscarinic receptors with little effect on nicotinic receptors. Distigmine bromide is an acetylcholinesterase inhibitor that inhibits the acetylcholinesterase enzyme from deactivating acetylcholine, thereby increasing both the level and duration of action of acetylcholine in neuromuscular junctions. Considering their inherent pharmacological activity, both agents may well enhance detrusor contractility and have been widely used for UAB management for decades. However, clinical data show conflicting results (Table 1).
A systemic review by Barendrecht et al. [8] concluded that the evidence does not support the use of parasympathomimetics to treat UAB, specifically when potential adverse effects are taken into account. The potential adverse effects include nausea, vomiting, diarrhea, gastrointestinal cramps, bronchospasms, salivation, sweating, headache, flushing, visual accommodation defect, and the rare but potentially lethal complication of cardiac arrest [9]. Because of the risk of potential adverse effects, clinicians tend to be reluctant to increase the dose of parasympathomimetics, which may contribute to unsatisfactory clinical efficacy. Along with the low-dose issue, a state of complete myogenic failure of the detrusor could be another probable reason for nonresponsiveness.
Riedl et al. [19] showed that electromotive administration of intravesical bethanechol was helpful for identifying a bethanechol-effective subgroup. The test consisted of an intravesical instillation of 20-mg bethanechol in 150 mL of 0.3% sodium chloride with and without a 20-mA current applied via an electrode catheter. In another study, the bladder electrical perception threshold was suggested to identify UAB patients likely to respond to bethanechol [20]. Increased bladder sensitivity as determined by an electrical perception threshold test indicates enhanced bladder emptying with subcutaneous injection of bethanechol.
Chronic bladder outlet obstruction (BOO) has been suggested as a risk factor for UAB. It has been well documented that experimental chronic BOO causes irreversible damage to the detrusor resulting in detrusor underactivity [2122]. Especially in elderly patients with a large prostate, BOO may play a main role in the development of UAB. Alpha-adrenergic blockers (ABs) have been widely used to relieve BOO and enhance bladder emptying in patients with neurogenic lower urinary tract dysfunction those with benign prostatic hyperplasia. Improvement of pelvic ischemia may contribute to the pharmacological effect of ABs in voiding dysfunction [23242526].
However, it is still unclear whether the clinical benefit of ABs for UAB comes from modulating the bladder itself or from a hidden component of BOO. Chang et al. [27] reported the efficacy of ABs in female patients with voiding difficulty and a subnormal maximal flow rate (MFR). After 6 weeks of therapy with 0.2-mg tamsulosin, a good therapeutic response, defined as achieving more than a 50% decrease in voiding symptom score and more than a 30% increase in MFR, was observed in 35.1% of the patients. In the subgroup analysis based on urodynamic study (UDS), a good therapeutic response was observed in 39.4% of patients with BOO and 32.7% of patients with UAB [27].
Combination therapy with cholinomimetics and ABs was shown to be more effective than monotherapy in patients with UAB [18]. The patients were assigned to 3 groups: a cholinomimetics group taking bethanechol chloride (60 mg/d) or distigmine bromide (15 mg/d); an AB group taking urapidil (60 mg/d); and a combination group taking both medications. After 4 weeks, International Prostate Symptom Score (IPSS) and PVRU were significantly decreased in the AB group and the combination group while they remained unchanged in the cholinomimetics group. The average and maximum flow rates significantly increased only in the combination group. PVRU decreased significantly in the AB group (p=0.0043) and the combination group (p=0.0008).
The efficacy of ABs has not yet been clearly demonstrated and the level of evidence remains weak. However, selective ABs may contribute to enhancing voiding efficiency and decreasing possible upper urinary tract damage. ABs may well be considered as an acceptable initial therapy in patients with UAB and chronic retention.
Prostaglandin E2 (PGE2) is a naturally occurring prostaglandin which functions as a direct vasodilator and smooth muscle relaxer. It also inhibits the release of noradrenaline from sympathetic nerve terminals. Experimental studies have shown that PGE2 can stimulate detrusor contraction directly, enhance the efficacy of other contraction-mediating transmitters, and potentiate afferent transmissions [28].
Andersson et al. [29] reported that intravesical PGE2 induces a significant increase in intravesical pressure and a decrease in maximum urethral closing pressure. In a prospective study of 36 patients with UAB, 72% showed improvement in detrusor function after intravesical PGE2 administration [30]. A prolonged benefit was observed in 39%, especially in patients with an intact sacral reflex arc, in patients with urodynamic evidence of a pathologically enlarged bladder, and in patients without outflow obstruction.
However, in a prospectively randomized double-blind study of 28 patients with urinary retention after anterior colporrhaphy, intravesical PGE2 on postoperative days 6 and 7 showed no significant effect on PVRU compared with placebo [31]. Combination therapy of intravesical PGE2 and oral bethanechol chloride showed limited benefit compared with the placebo combination [17]. Before treatment, the median PVRU was 426 mL for those receiving both drugs; this decreased to 325 mL after 6 weeks (p<0.015). In the placebo combination group, respective values were 576 and 538 mL (p=0.09). Only 4 of 9 patients in the active combination group reported symptomatic improvement and were able to reduce the frequency of clean intermittent catheterization.
Even though there was evidence of a pharmacological effect, PGE2 had a limited therapeutic effect compared with placebo in clinical trials. Therefore, intravesical PGE2 would not be recommend as a routine treatment, but may be considered as an additive therapeutic regimen in UAB patients performing clean intermittent catheterization or those with an indwelling catheter.
Acotiamide is an oral, first-in-class prokinetic drug that modulates upper gastrointestinal motility to alleviate abdominal symptoms resulting from hypomotility and delayed gastric emptying [32]. The pharmacological action of acotiamide exerts an antagonistic effect on muscarinic receptors to inhibit the negative feedback system by blocking the muscarinic auto receptors that regulate acetylcholine release. Hence, acotiamide enhances parasympathetic activity by increasing acetylcholine release as well as by inhibiting acetylcholinesterase activity.
Sugimoto et al. [33] conducted a small pilot study of 19 UAB patients treated with oral acotiamide. After 2 weeks of acotiamide hydrochloride hydrate at a dose of 100 mg 3 times daily, the drug was generally well tolerated with little adverse effect. PVRU showed a significant decrease from 161.4±90.0 mL at baseline to 116.3±63.1 mL at the end of the study (p=0.006). To further determine the clinical efficacy of acotiamide, a prospective randomized controlled study would be warranted in the near future.
Transurethral intravesical electrical stimulation (IVES) may activate specific mechanoreceptors and the intramural motor system in the detrusor, which sequentially leads to local muscle contractions. These contractions stimulate afferent pathways, the CNS, and efferent pathways, which make more coordinated and enhanced detrusor contractions. Theoretically, IVES can be applied in patients with an incomplete spinal cord lesion.
IVES has a long history of use in bladder rehabilitation to facilitate voiding and decrease PVRU in the management of neurogenic bladder associated with meningomyelocele [34]. Primus et al. [35] reported that detrusor contraction was achieved in 39%, bladder sensation in 75%, and catheter-free status in 54% of patients after IVES. Lombardi et al. [36] reported the efficacy of IVES in patients with incomplete spinal cord lesion with chronic neurogenic nonobstructive urinary retention. Significant predictive parameters for IVES success were a timespan of less than 2 years from the onset of spinal cord lesion and the presence of first sensation of bladder filling at baseline [36]. The relevant study results so far suggest that the clinical efficacy of IVES is rather short-term and tends to decrease with time [3637].
Sacral nerve modulation (SNM) modulates pelvic/perineal afferent pathways, which increase parasympathetic activity in the bladder while inhibiting the sympathetic urethral and somatic sphincter components in guarding the reflex to relax outlet [37]. Therefore, an intact neural circuit for the voiding reflex is mandatory to achieve clinical efficacy from SNM.
Lombardi et al. [38] compared IVES and SNM in 77 patients with incomplete spinal cord lesion and neurogenic nonobstructive urinary retention. After sequential test stimulation, 29 patients (37.6%) responded to both IVES and first-stage SNM. Whereas all IVES responders returned to the baseline state after 9.6 months of mean follow-up, only 34.5% of the responders became nonresponsive to permanent SNM after 54 months of mean follow-up. Permanent SNM showed a significantly longer clinical benefit than IVES [38]. In another study, 36 of 85 patients (42.4%) classified as responders after first-stage SNM, and 34 patients proceeded to permanent SNM. Eleven inconstant responders who returned to baseline at follow-up responded again with an implant on the contralateral S3 sacral root. Two patients who failed twice responded to an S4 sacral root implant. Only one failure was reported after more than 3 years of follow-up after permanent SNM [39].
In a multicenter, prospective randomized controlled study of patients with idiopathic urinary retention refractory to standard therapy, 68 of 177 patients (38.4%) had more than 50% improvement after first-stage SNM [40]. In 37 patients, permanent SNM proceeded immediately and voluntary voiding was restored in 69% of the patients at 6 months. An additional 14% of patients had a more than 50% reduction in catheterized volume per catheterization. Overall successful results were reported in 83% of the SNM group compared with 9% of the control group at 6 months. Temporary inactivation of the implant resulted in a significant increase in PVRU, and the efficacy of SNM was sustained until 18 months of follow-up.
In 1999, the U.S. Food and Drug Administration (FDA) approved the Medtronic Interstim System for Urinary Control, an implantable electrostimulation device for SNM, for the treatment of urinary retention and refractory overactive bladder. So far, SNM is the only FDA-approved surgical management option for nonobstructive UAB.
As stated earlier, chronic BOO may lead to UAB. Surgery to reduce BOO, such as transurethral resection of the prostate (TURP), has been regarded as a viable management option for UAB combined with BOO. Many researchers have reported favorable short-term results of TURP [414243], prostatectomy with laser energy [4344454647], transurethral incision of the bladder neck [4849], or onabotulinumtoxinA injection on the bladder neck [5051] for patients with both BOO and UAB.
However, long-term studies have shown conflicting results on the effectiveness of BOO reduction surgery. Masumori et al. [52] reported that patients showed significantly better IPSS at 12 years after TURP than at baseline, although there was gradual deterioration with time. In patients without BOO, the IPSS deteriorated faster than in those with BOO, while neither UAB nor detrusor overactivity influenced the degree of change in IPSS. The quality of life index remained improved regardless of preoperative UDS findings. Noticeably, two-thirds of patients with UAB without BOO remained satisfied at 12 years. The results may be subject to bias, because only 47% of the initial patients were surviving at the time of the final evaluation [52]. In a 10-year follow-up study, Al-Hayek et al. [53] reported no significant change in the bladder contractility index (BCI) in patients with BOO after TURP. Also, the BCI in patients with untreated UAB showed no significant change. Noticeably, the BCI was higher in untreated patients with BOO than in the TURP group at the time of the final evaluation. Thomas et al. [54] also reported that no significant clinical improvement was observed in patients with UAB at 11 years of follow-up. Moreover, UAB patients with TURP showed significantly higher PVRU than did those without surgery.
A recent meta-analysis showed that the presence of UAB correlated with poorer IPSS and MFR improvement after surgery in patients with benign prostatic hyperplasia and UAB [55]. Although surgery to reduce BOO may be a viable treatment option in men with presumed BOO and reduced detrusor contractility, acontractile bladder usually indicates a poor prognostic sign [56]. In real clinical practice, there seems to be little role of surgery to reduce outlet resistance in patients with UAB but without BOO.
Chronic urinary retention associated with BOO and/or detrusor underactivity commonly progresses to myogenic decompensation with rather large bladder capacity [57]. In a large decompensated bladder with detrusor underactivity without BOO, decreasing the large bladder capacity may be helpful to enhance emptying.
The theoretical background for reduction cystoplasty lies in the bladder biomechanics based on the Laplace law [58]. When applied to the bladder, the Laplace equation can be described as T=Pves×R/2d, where T is wall tension, P is intravesical pressure, R is the radius of the bladder, and d is wall thickness [59]. The equation indicates a direct relationship between wall tension and bladder diameter. If the bladder diameter is decreased by half after reduction cystoplasty, the wall tension can double under constant intravesical pressure.
Several surgical techniques for reduction cystoplasty have been suggested in the literature: fundus invagination (Stewart technique) [60], detrusor wrap (Zoedler technique) [61], and transection and resection of the superior bladder dome (Klarskov technique) [62]. However, the actual clinical outcomes for these techniques did not parallel the theoretical background. In a long-term follow-up report after reduction cystoplasty, bladder capacity and PVRU tended to increase over time [63]. Although the results were from a small series with mixed pathology and a different surgical approach, more favorable outcomes were reported in patients with hypocontractile detrusor function compared those with an acontractile detrusor [6264].
A criticism of reduction cystoplasty is that it may not increase detrusor contractility but rather only decrease bladder compliance, which may put the patient at risk of upper tract damage and overflow incontinence. Therefore, reduction cystoplasty has limited clinical feasibility in patients with UAB and should be considered very carefully in selected cases with some residual detrusor contractility.
The concept for this surgical approach comes from the idea that voiding efficiency in UAB may be facilitated by wrapping the bladder with striated muscle to increase intravesical pressure. A rectus abdominis wrap was applied in early series but the functional results were unsatisfactory. The movement of the covered rectus failed to generate an effective downward force to compress the bladder. The other problem was the segmental innervation, which denervated most of the rectus after a major dissection [65].
The latissimus dorsi (LD) is a large, flat muscle on the back that is innervated by the thoracodorsal nerve. The muscle is a potential source for breast reconstruction after mastectomy [66] or dynamic cardiomyoplasty [67]. Dynamic cardiomyoplasty consists of wrapping the LD around the heart and electrostimulating the muscle in synchrony with ventricular systole. Latissimus dorsi detrusor myoplasty (LDDM) consists of wrapping the free LD muscle flap around the bladder and making a neurovascular anastomosis to the lowest motor branches of the intercostal nerve and deep inferior epigastric vessels.
On the basis of animal experiments [68], LDDM was first applied in 1998 in 3 patients with acontractile bladder requiring catheterization [69]. UDS at 12 months after LDDM showed restoration of self-voiding in all patients with an acceptable range of MFR (18–26 mL/s) and PVRU (0–90 mL). Contraction of the transplanted muscle was confirmed by ultrasonography and flow-mode computerized tomography [69].
A subsequent study reported that voluntary self-voiding was restored in 14 of 20 acontractile bladder patients (70%) with PVRU less than 100 mL [70]. Another four patients showed voluntary voiding after additional bladder neck incision. Mean postoperative detrusor pressure was 72 cmH2O. Functioning free muscle transplantation, LDDM, restored voluntary voiding in 90% of patients previously on long-term catheterization.
The first worldwide multicenter study of LDDM for acontractile bladder from lower motor neuron lesions showed restoration of voluntary voiding in 17 of 24 patients (71%) with a mean PVRU of 25 mL and a significant increase in the mean BCI [71]. In 3 patients (13%), the number of catheterizations was reduced to 2 to 4 times daily with a mean PVRU of 200 mL [71]. Idiopathic acontractile bladder in elderly patients showed a poor outcome, and it is suggested that special caution be taken in patients with no clear origin of bladder acontractility.
LDDM is an extensive and invasive surgery that requires a multidisciplinary team approach of urologists and plastic reconstructive experts in functional reconstruction. Although the results are based on a rather small number of studies, LDDM shows promise for restoring voluntary voiding especially in motivated young patients who hope to live a life without catheterization.
The current management of UAB remains unsatisfactory. Normal bladder emptying requires normal sensation, intact CNS control, and normal detrusor neurotransmission and contractility; derangements in any of these processes may result in UAB. The multifactorial nature of UAB pathogenesis complicates the appropriate management for each patient. A specific management strategy that focuses on only one aspect of UAB may not provide adequate therapeutic efficacy. Future research to establish a more clinically relevant definition of UAB will be required to open a new era of UAB management.
References
1. Andersson KE. The many faces of impaired bladder emptying. Curr Opin Urol. 2014; 24:363–369. PMID: 24752226.

2. Andersson KE. Detrusor underactivity/underactive bladder: new research initiatives needed. J Urol. 2010; 184:1829–1830. PMID: 20846686.

3. Miyazato M, Yoshimura N, Chancellor MB. The other bladder syndrome: underactive bladder. Rev Urol. 2013; 15:11–22. PMID: 23671401.
4. Thomas AW, Cannon A, Bartlett E, Ellis-Jones J, Abrams P. The natural history of lower urinary tract dysfunction in men: minimum 10-year urodynamic follow-up of untreated detrusor underactivity. BJU Int. 2005; 96:1295–1300. PMID: 16287448.

5. Consortium for Spinal Cord Medicine. Outcomes following traumatic spinal cord injury: clinical practice guidelines for health-care professionals. J Spinal Cord Med. 2000; 23:289–316. PMID: 17536300.
6. El-Masri WS, Chong T, Kyriakider AE, Wang D. Long-term follow-up study of outcomes of bladder management in spinal cord injury patients under the care of the Midlands Centre for Spinal Injuries in Oswestry. Spinal Cord. 2012; 50:14–21. PMID: 21808256.

7. Aquilonius SM, Hartvig P. Clinical pharmacokinetics of cholinesterase inhibitors. Clin Pharmacokinet. 1986; 11:236–249. PMID: 3524957.

8. Barendrecht MM, Oelke M, Laguna MP, Michel MC. Is the use of parasympathomimetics for treating an underactive urinary bladder evidence-based? BJU Int. 2007; 99:749–752. PMID: 17233798.

9. Taylor P. Anticholinesterase agents. Goodman LD, Gilman A, Brunton LL, Lazo JS, Parker KL. Goodman and Gilman's the pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill;2006. p. 211–212.
10. Fleming AR. The use of urecholine in the prevention of postpartum urinary retention; final report. Am J Obstet Gynecol. 1957; 74:569–571. PMID: 13458255.
11. Barrett DM. The effect of oral bethanechol chloride on voiding in female patients with excessive residual urine: a randomized double-blind study. J Urol. 1981; 126:640–642. PMID: 6117662.

12. Shah PJ, Abrams PH, Choa RG, Ashken MH, Gaches CG, Green NA, et al. Distigmine bromide and post-prostatectomy voiding. Br J Urol. 1983; 55:229–232. PMID: 6132645.

13. Gottesman L, Milsom JW, Mazier WP. The use of anxiolytic and parasympathomimetic agents in the treatment of postoperative urinary retention following anorectal surgery. A prospective, randomized, double-blind study. Dis Colon Rectum. 1989; 32:867–870. PMID: 2571469.
14. Savona-Ventura C, Grech ES, Saliba I. Pharmacological measures to prevent post-operative urinary retention; a prospective randomized study. Eur J Obstet Gynecol Reprod Biol. 1991; 41:225–229. PMID: 1682174.

15. Kemp B, Kitschke HJ, Goetz M, Heyl W. Prophylaxis and treatment of bladder dysfunction after Wertheim-Meigs operation: the positive effect of early postoperative detrusor stimulation using the cholinergic drug betanecholchloride. Int Urogynecol J Pelvic Floor Dysfunct. 1997; 8:138–141. PMID: 9449585.

16. Burger DH, Kappetein AP, Boutkan H, Breslau PJ. Prevention of urinary retention after general surgery: a controlled trial of carbachol/diazepam versus alfusozine. J Am Coll Surg. 1997; 185:234–236. PMID: 9291399.

17. Hindley RG, Brierly RD, Thomas PJ. Prostaglandin E2 and bethanechol in combination for treating detrusor underactivity. BJU Int. 2004; 93:89–92. PMID: 14678375.

18. Yamanishi T, Yasuda K, Kamai T, Tsujii T, Sakakibara R, Uchiyama T, et al. Combination of a cholinergic drug and an alpha-blocker is more effective than monotherapy for the treatment of voiding difficulty in patients with underactive detrusor. Int J Urol. 2004; 11:88–96. PMID: 14706012.

19. Riedl CR, Stephen RL, Daha LK, Knoll M, Plas E, Pflüger H. Electromotive administration of intravesical bethanechol and the clinical impact on acontractile detrusor management: introduction of a new test. J Urol. 2000; 164:2108–2111. PMID: 11061937.

20. De Wachter S, Van Meel TD, Wyndaele JJ. Study of the afferent nervous system and its evaluation in women with impaired detrusor contractility treated with bethanechol. Urology. 2003; 62:54–58. PMID: 12837422.

21. Levin RM, Longhurst PA, Barasha B, McGuire EJ, Elbadawi A, Wein AJ. Studies on experimental bladder outlet obstruction in the cat: long-term functional effects. J Urol. 1992; 148:939–943. PMID: 1512863.

22. Haugaard N, Potter L, Wein AJ, Levin RM. Effect of partial obstruction of the rabbit urinary bladder on malate dehydrogenase and citrate synthase activity. J Urol. 1992; 147:1391–1393. PMID: 1485895.

23. Moon KH, Park CH, Jung HC, Oh TH, Kim JS, Kim DY. A 12-week, open label, multi-center study to evaluate the clinical efficacy and safety of silodosin on voiding dysfunction in patients with neurogenic bladder. Low Urin Tract Symptoms. 2015; 7:27–31. PMID: 26663648.

24. Andersson KE, Nomiya M, Yamaguchi O. Chronic pelvic ischemia: contribution to the pathogenesis of lower urinary tract symptoms (LUTS): a new target for pharmacological treatment? Low Urin Tract Symptoms. 2015; 7:1–8. PMID: 26663644.

25. Nomiya M, Andersson KE, Yamaguchi O. Chronic bladder ischemia and oxidative stress: new pharmacotherapeutic targets for lower urinary tract symptoms. Int J Urol. 2015; 22:40–46. PMID: 25339506.

26. Abrams P, Amarenco G, Bakke A, Buczyński A, Castro-Diaz D, Harrison S, et al. Tamsulosin: efficacy and safety in patients with neurogenic lower urinary tract dysfunction due to suprasacral spinal cord injury. J Urol. 2003; 170(4 Pt 1):1242–1251. PMID: 14501734.

27. Chang SJ, Chiang IN, Yu HJ. The effectiveness of tamsulosin in treating women with voiding difficulty. Int J Urol. 2008; 15:981–985. PMID: 18721208.

28. Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004; 56:581–631. PMID: 15602011.

29. Andersson KE, Henriksson L, Ulmsten U. Effects of prostaglandin E2 applied locally on intravesical and intraurethral pressures in women. Eur Urol. 1978; 4:366–369. PMID: 568550.
30. Desmond AD, Bultitude MI, Hills NH, Shuttleworth KE. Clinical experience with intravesical prostaglandin E2. A prospective study of 36 patients. Br J Urol. 1980; 52:357–366. PMID: 7427009.
31. Wagner G, Husslein P, Enzelsberger H. Is prostaglandin E2 really of therapeutic value for postoperative urinary retention? Results of a prospectively randomized double-blind study. Am J Obstet Gynecol. 1985; 151:375–379. PMID: 3881968.

32. Doi Y, Murasaki O, Kaibara M, Uezono Y, Hayashi H, Yano K, et al. Characterization of functional effects of Z-338, a novel gastroprokinetic agent, on the muscarinic M1, M2, and M3 receptors expressed in Xenopus oocytes. Eur J Pharmacol. 2004; 505:31–35. PMID: 15556134.

33. Sugimoto K, Akiyama T, Shimizu N, Matsumura N, Hayashi T, Nishioka T, et al. A pilot study of acotiamide hydrochloride hydrate in patients with detrusor underactivity. Res Rep Urol. 2015; 7:81–83. PMID: 26056686.

34. Dénes J, Léb J. Electrostimulation of the neuropathic bladder. J Pediatr Surg. 1975; 10:245–247. PMID: 1091723.

35. Primus G, Kramer G, Pummer K. Restoration of micturition in patients with acontractile and hypocontractile detrusor by transurethral electrical bladder stimulation. Neurourol Urodyn. 1996; 15:489–497. PMID: 8857617.

36. Lombardi G, Celso M, Mencarini M, Nelli F, Del Popolo G. Clinical efficacy of intravesical electrostimulation on incomplete spinal cord patients suffering from chronic neurogenic non-obstructive retention: a 15-year single centre retrospective study. Spinal Cord. 2013; 51:232–237. PMID: 23147136.

37. Leng WW, Chancellor MB. How sacral nerve stimulation neuromodulation works. Urol Clin North Am. 2005; 32:11–18. PMID: 15698871.

38. Lombardi G, Musco S, Celso M, Ierardi A, Nelli F, Del Corso F, et al. Intravesical electrostimulation versus sacral neuromodulation for incomplete spinal cord patients suffering from neurogenic non-obstructive urinary retention. Spinal Cord. 2013; 51:571–578. PMID: 23628893.

39. Lombardi G, Musco S, Celso M, Del Corso F, Del Popolo G. Sacral neuromodulation for neurogenic non-obstructive urinary retention in incomplete spinal cord patients: a ten-year follow-up single-centre experience. Spinal Cord. 2014; 52:241–245. PMID: 24394604.

40. Jonas U, Fowler CJ, Chancellor MB, Elhilali MM, Fall M, Gajewski JB, et al. Efficacy of sacral nerve stimulation for urinary retention: results 18 months after implantation. J Urol. 2001; 165:15–19. PMID: 11125353.

41. Seki N, Kai N, Seguchi H, Takei M, Yamaguchi A, Naito S. Predictives regarding outcome after transurethral resection for prostatic adenoma associated with detrusor underactivity. Urology. 2006; 67:306–310. PMID: 16461081.

42. Sokhal AK, Sinha RJ, Purkait B, Singh V. Transurethral resection of prostate in benign prostatic enlargement with underactive bladder: a retrospective outcome analysis. Urol Ann. 2017; 9:131–135. PMID: 28479762.

43. Woo MJ, Ha YS, Lee JN, Kim BS, Kim HT, Kim TH, et al. Comparison of surgical outcomes between holmium laser enucleation and transurethral resection of the prostate in patients with detrusor underactivity. Int Neurourol J. 2017; 21:46–52. PMID: 28361512.

44. Cho MC, Ha SB, Park J, Son H, Oh SJ, Kim SW, et al. Impact of detrusor underactivity on surgical outcomes of laser prostatectomy: comparison in serial 12-month follow-up outcomes between potassium-titanyl-phosphate photoselective vaporization of the prostate (PVP) and holmium laser enucleation of the prostate (HoLEP). Urology. 2016; 91:158–166. PMID: 26879733.

45. Cho MC, Park J, Kim JK, Cho SY, Jeong H, Oh SJ, et al. Can preoperative detrusor underactivity influence surgical outcomes of 120 W HPS vaporization of the prostate (PVP) or holmium laser enucleation of the prostate (HoLEP)? A serial 3-year follow-up study. Neurourol Urodyn. 2017; 6. 09. DOI: 10.1002/nau.23317. [Epub].
46. Lomas DJ, Krambeck AE. Long-term efficacy of holmium laser enucleation of the prostate in patients with detrusor underactivity or acontractility. Urology. 2016; 97:208–211. PMID: 27450935.

47. Yu Z, Li J, Li Z, Hou R. Photoselective vaporization of the prostate and simultaneous suprapubic cystostomy for the treatment of benign prostatic hyperplasia in patients with mild to severe detrusor underactivity. Urol Int. 2015; 95:269–275. PMID: 26138113.

48. Jhang JF, Jiang YH, Kuo HC. Transurethral incision of the bladder neck improves voiding efficiency in female patients with detrusor underactivity. Int Urogynecol J. 2014; 25:671–676. PMID: 24288115.

49. Jhang JF, Jiang YH, Lee CL, Kuo HC. Long-term follow up and predictive factors for successful outcome of transurethral incision of the bladder neck in women with detrusor underactivity. J Formos Med Assoc. 2016; 115:807–813. PMID: 26375777.

50. Phelan MW, Franks M, Somogyi GT, Yokoyama T, Fraser MO, Lavelle JP, et al. Botulinum toxin urethral sphincter injection to restore bladder emptying in men and women with voiding dysfunction. J Urol. 2001; 165:1107–1110. PMID: 11257648.

51. Mokhless I, Gaafar S, Fouda K, Shafik M, Assem A. Botulinum A toxin urethral sphincter injection in children with nonneurogenic neurogenic bladder. J Urol. 2006; 176(4 Pt 2):1767–1770. PMID: 16945643.

52. Masumori N, Furuya R, Tanaka Y, Furuya S, Ogura H, Tsukamoto T. The 12-year symptomatic outcome of transurethral resection of the prostate for patients with lower urinary tract symptoms suggestive of benign prostatic obstruction compared to the urodynamic findings before surgery. BJU Int. 2010; 105:1429–1433. PMID: 19863522.

53. Al-Hayek S, Thomas A, Abrams P. Natural history of detrusor contractility--minimum ten-year urodynamic follow-up in men with bladder outlet obstruction and those with detrusor. Scand J Urol Nephrol Suppl. 2004; (215):101–108. PMID: 15545204.
54. Thomas AW, Cannon A, Bartlett E, Ellis-Jones J, Abrams P. The natural history of lower urinary tract dysfunction in men: the influence of detrusor underactivity on the outcome after transurethral resection of the prostate with a minimum 10-year urodynamic follow-up. BJU Int. 2004; 93:745–750. PMID: 15049984.

55. Kim M, Jeong CW, Oh SJ. Effect of preoperative urodynamic detrusor underactivity on transurethral surgery for benign prostatic hyperplasia: a systematic review and meta-analysis. J Urol. 2017; 7. 29. [Epub]. pii: S0022-5347(17)77237-0. DOI: 10.1016/j.juro.2017.07.079.
56. Choi SW, Choi YS, Bae WJ, Kim SJ, Cho HJ, Hong SH, et al. 120 W Greenlight HPS laser photoselective vaporization of the prostate for treatment of benign prostatic hyperplasia in men with detrusor underactivity. Korean J Urol. 2011; 52:824–828. PMID: 22216394.

57. Weir J, Jaques PF. Large-capacity bladder. A urodynamic survey. Urology. 1974; 4:544–548. PMID: 4428554.

58. Chancellor MB, Rivas DA, Bourgeois IM. Laplace's law and the risks and prevention of bladder rupture after enterocystoplasty and bladder autoaugmentation. Neurourol Urodyn. 1996; 15:223–233. PMID: 8732989.

59. Yoshimura N, Chancellor MB. Physiology and pharmacology of the bladder and urethra. In : Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh urology. 10th. Philadelphia (PA): Elsevier/Saunders;2012. p. 1786–1833.
60. Stewart HH. The surgical treatment of severe chronic retention without large diverticula. Br J Urol. 1966; 38:685–695. PMID: 5958051.
61. Zoedler D. Surgical treatment of bladder atony. Z Urol. 1964; 57:743–748. PMID: 14318438.
62. Klarskov P, Holm-Bentzen M, Larsen S, Gerstenberg T, Hald T. Partial cystectomy for the myogenic decompensated bladder with excessive residual urine. Urodynamics, histology and 2-13 years follow-up. Scand J Urol Nephrol. 1988; 22:251–256. PMID: 3238329.
63. Bukowski TP, Perlmutter AD. Reduction cystoplasty in the prune belly syndrome: a long-term followup. J Urol. 1994; 152(6 Pt 1):2113–2116. PMID: 7966698.

64. Kinn AC. The lazy bladder: appraisal of surgical reduction. Scand J Urol Nephrol. 1985; 19:93–99. PMID: 4059883.
65. Zhang YH, Shao QA, Wang JM. Enveloping the bladder with displacement of flap of the rectus abdominis muscle for the treatment of neurogenic bladder. J Urol. 1990; 144:1194–1195. PMID: 2146404.
66. Blackburn NE, Mc Veigh JG, Mc Caughan E, Wilson IM. The musculoskeletal consequences of breast reconstruction using the latissimus dorsi muscle for women following mastectomy for breast cancer: a critical review. Eur J Cancer Care (Engl). 2017; 2. 10. [Epub]. DOI: 10.1111/ecc.12664.

67. Dumcius A, Chekanov V, Vysockas V. Current status of cardiomyoplasty as surgical alternative for end-stage heart failure. Medicina (Kaunas). 2003; 39:815–822. PMID: 14515042.
68. Stenzl A, Ninkovic M, Willeit J, Hess M, Feichtinger H, Schwabegger A, et al. Free neurovascular transfer of latissimus dorsi muscle to the bladder. I. Experimental studies. J Urol. 1997; 157:1103–1108. PMID: 9072552.

69. Stenzl A, Ninkovic M, Kölle D, Knapp R, Anderl H, Bartsch G. Restoration of voluntary emptying of the bladder by transplantation of innervated free skeletal muscle. Lancet. 1998; 351:1483–1485. PMID: 9605805.

70. Ninkovic M, Stenzl A, Schwabegger A, Bartsch G, Prosser R, Ninkovic M. Free neurovascular transfer of latisstmus dorsi muscle for the treatment of bladder acontractility: II. Clinical results. J Urol. 2003; 169:1379–1383. PMID: 12629366.
71. Gakis G, Ninkovic M, van Koeveringe GA, Raina S, Sturtz G, Rahnama'i MS, et al. Functional detrusor myoplasty for bladder acontractility: long-term results. J Urol. 2011; 185:593–599. PMID: 21168866.

Table 1
Randomized controlled trials of parasympathomimetics for underactive bladder
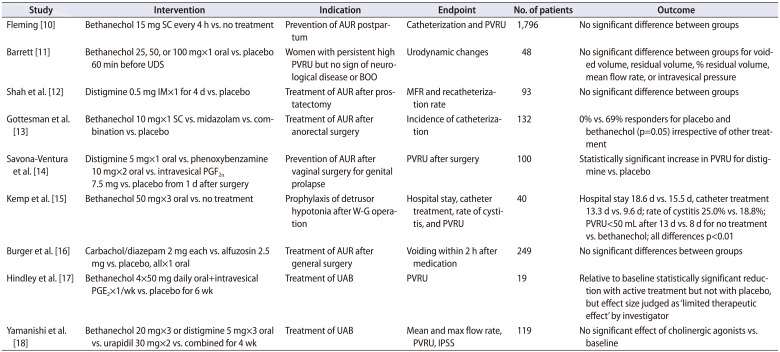
| Study | Intervention | Indication | Endpoint | No. of patients | Outcome |
|---|---|---|---|---|---|
| Fleming [10] | Bethanechol 15 mg SC every 4 h vs. no treatment | Prevention of AUR postpartum | Catheterization and PVRU | 1,796 | No significant difference between groups |
| Barrett [11] | Bethanechol 25, 50, or 100 mg×1 oral vs. placebo 60 min before UDS | Women with persistent high PVRU but no sign of neurological disease or BOO | Urodynamic changes | 48 | No significant difference between groups for voided volume, residual volume, % residual volume, mean flow rate, or intravesical pressure |
| Shah et al. [12] | Distigmine 0.5 mg IM×1 for 4 d vs. placebo | Treatment of AUR after prostatectomy | MFR and recatheterization rate | 93 | No significant difference between groups |
| Gottesman et al. [13] | Bethanechol 10 mg×1 SC vs. midazolam vs. combination vs. placebo | Treatment of AUR after anorectal surgery | Incidence of catheterization | 132 | 0% vs. 69% responders for placebo and bethanechol (p=0.05) irrespective of other treatment |
| Savona-Ventura et al. [14] | Distigmine 5 mg×1 oral vs. phenoxybenzamine 10 mg×2 oral vs. intravesical PGF2α 7.5 mg vs. placebo from 1 d after surgery | Prevention of AUR after vaginal surgery for genital prolapse | PVRU after surgery | 100 | Statistically significant increase in PVRU for distigmine vs. placebo |
| Kemp et al. [15] | Bethanechol 50 mg×3 oral vs. no treatment | Prophylaxis of detrusor hypotonia after W-G operation | Hospital stay, catheter treatment, rate of cystitis, and PVRU | 40 | Hospital stay 18.6 d vs. 15.5 d, catheter treatment 13.3 d vs. 9.6 d; rate of cystitis 25.0% vs. 18.8%; PVRU<50 mL after 13 d vs. 8 d for no treatment vs. bethanechol; all differences p<0.01 |
| Burger et al. [16] | Carbachol/diazepam 2 mg each vs. alfuzosin 2.5 mg vs. placebo, all×1 oral | Treatment of AUR after general surgery | Voiding within 2 h after medication | 249 | No significant differences between groups |
| Hindley et al. [17] | Bethanechol 4×50 mg daily oral+intravesical PGE2×1/wk vs. placebo for 6 wk | Treatment of UAB | PVRU | 19 | Relative to baseline statistically significant reduction with active treatment but not with placebo, but effect size judged as ‘limited therapeutic effect’ by investigator |
| Yamanishi et al. [18] | Bethanechol 20 mg×3 or distigmine 5 mg×3 oral vs. urapidil 30 mg×2 vs. combined for 4 wk | Treatment of UAB | Mean and max flow rate, PVRU, IPSS | 119 | No significant effect of cholinergic agonists vs. baseline |
SC, subcutaneous; AUR, acute urinary retention; UDS, urodynamic study; PVRU, postvoid residual urine volume; BOO, bladder outlet obstruction; IM, intramuscular; MFR, maximal flow rate; PGF2, prostaglandin F2; PGE2, prostaglandin E2; W–G, Wertheim-Meigs; UAB, underactive bladder; IPSS, International Prostate Symptom Score.
Modified from Barendrecht et al. BJU Int 2007;99:749-52 [8], with permission of John Wiley & Sons.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download