Abstract
Chronic rhinosinusitis (CRS) is one of the most common presentations of upper airway illness and severely affects patient quality of life. Its frequency is not surprising given levels of environmental exposure to microbes, pollutants, and allergens. Inflammatory cells, inflammatory cytokine and chemokine production, and airway remodeling have been detected in the sinonasal mucosae of CRS patients, although the precise pathophysiological mechanisms causing such persistent inflammation remain unclear. Given its high prevalence and considerable associated morbidity, continued research into CRS is necessary to increase our understanding of factors likely to contribute to its pathogenesis, and facilitate the development of novel therapeutic strategies to improve treatment. The purpose of this review is to summarize the current state of knowledge regarding immune cell responses and epithelial alterations in CRS.
Chronic rhinosinusitis (CRS) is a common upper airway disease that affects 5 to 16% of the global population and has a significant socioeconomic impact. CRS is characterized by inflammation of the sinonasal mucosa for longer than 12 weeks, and is clinically associated with nasal obstruction, loss of sense of smell, nasal drainage, and facial pain. CRS can be subdivided into two major categories based on the presence (CRSwNP) or absence (CRSsNP) of nasal polyps (NPs) (12). Many contributing factors have been identified, including allergic responses, bacterial and fungal colonization, biofilm formation, the superantigen effect, immune dysfunction, impaired epithelial defense, and environmental exposures. Recently, defects in the innate immune function of the upper airway epithelium have been reported to play a role in the initial inflammatory response leading to CRS. Subsequent recruitment and activation of eosinophils, neutrophils, mast cells, basophils, and innate lymphoid cells further contributes to a chronic inflammatory response and directly activates adaptive immune cells, including T and B lymphocytes (34). CRSsNP is histologically characterized by basement membrane thickening, excessive numbers of goblet cells, submucosal gland hyperplasia and hypertrophy, and fibrosis. In contrast, CRSwNP is distinguished by stromal tissue edema, formation of pseudocysts, and significant immune cell infiltrate. However, the precise pathophysiological mechanisms that cause persistent, exaggerated sinonasal inflammation remain to be elucidated. Further studies are required to explain the high proportion of patients with CRS refractory to current conventional pharmacologic treatment. Recent advancements in our understanding of this disease's pathogenesis are highlighted in this review, with a special focus on immune cell responses and epithelial disruptions.
CRS has been intensively investigated over the past several decades. CRSwNP, one of the most extensively studied CRS subtypes, is typically associated with a type 2 inflammatory environment in Western countries. Th2 inflammation is characterized by infiltration of eosinophils, mast cells, basophils, and lymphocytes. Compared to NPs from Western patients, those from Asian patients are marked by reduced eosinophil numbers, and decreased levels of IL-5, eotaxin, and eosinophil cationic protein. This latter has been established as a standardized marker of tissue eosinophilia and eosinophil activation (5). Eosinophilic NPs have been recorded in less than 50% of CRSwNP patients in Asian countries (678). Although these studies suggest that eosinophilic NPs are less common in East Asia, some investigations have demonstrated a higher frequency of eosinophilia in NPs among Chinese patients (9). The discrepancy between these results may be explained by the fact that each research group employed a different system to identify eosinophilia (6910).
The inflammatory patterns of CRSsNP and CRSwNP are known to be different. Although unique characteristics distinguishing CRSsNP from other CRS subtypes remain unconfirmed, this subtype is thought to predominantly involve type 1 inflammation with higher IFN-γ and lower IL-5 expression than that observed in NPs of CRSwNP patients (1112). Researchers in Belgium, China, and Korea have reported higher IFN-γ levels in patients with CRSsNP than those with CRSwNP (61113). However, this classification has been under reconsideration, with several recent studies suggesting that IFN-γ expression is similar in NPs from individuals with CRSwNP and CRSsNP and control subjects (1415). Together, these findings suggest that CRSsNP may not necessarily imply greater type 1 inflammation. However, variations in protein expression levels between regions of the sinonasal cavity might explain the discrepancies in IFN-γ expression observed among NPs and other tissues. It has also been discovered that levels of TGF-β, a key inducer of fibrosis and airway remodeling, tend to be elevated in CRSsNP in European and Asian countries (161718). Interestingly, there seems to be some variation in TGF-β expression between locations within the sinonasal cavity, with the inferior and middle turbinates exhibiting the lowest levels in CRSsNP patients (19).
In Western populations, CRSwNP is often characterized by type 2 inflammation with elevated levels of Th2 cytokines such as IL-4, IL-5, and IL-13 in sinonasal tissue, along with eosinophilia (112021). Accumulating evidence from Asian countries, especially China, Korea, and Japan, shows that CRSwNP in these populations may be characterized by a mixed Th1/Th17 inflammatory pattern, with greater neutrophilic inflammation than that observed in NPs from patients of Western countries (6722). Notably, recent studies have demonstrated that the prevalence of eosinophilic NPs is increasing in Asia. For instance, in a study based in Thailand, Katotomichelakis et al. evaluated NPs at two time points, 1999 and 2011, finding that the average number of eosinophils per high power field increased from 5 to 35 over this period (23). Moreover, Kim et al . found that the prevalence of eosinophilic NPs in Korea has increased to 50.9% (10). Owing to the uncertainty concerning inflammatory patterns of CRS subtypes, several researchers have proposed new classification strategies. For example, it has been suggested that CRS could be subdivided based on histopathological findings into chronic hyperplastic eosinophilic sinusitis or chronic inflammatory sinusitis (defined as CRS without evidence of tissue eosinophilia). Using this approach, NPs can be associated with one of these two conditions (5).
Compared to those exhibiting eosinophilic inflammation, inflammatory patterns in non-eosinophilic NPs have been less thoroughly investigated. Non-eosinophilic NPs can be divided into several phenotypes, of which the neutrophilic phenotype is the best characterized. The mechanisms that drive these unique regional phenotypic differences are not yet clear, although recent studies suggest that a genetic component may be involved. Larger investigations of the general population are needed to investigate the factors that contribute to CRS pathogenesis and morbidity.
Numerous studies have been carried out to examine the role of innate immune cells in CRS pathology. Eosinophils play a key part, although many other immune cell types also have important functions in the pathogenesis of this condition. Eosinophils and other immune cells are recruited to the nasal cavity in patients with CRS, where they produce inflammatory mediators and induce pathologic changes in the respiratory mucosa. There are many factors that influence the production, migration, survival, and death of eosinophils (24). Expression of the cytokine IL-5 appears to be particularly important for these cells (25). In 1997, IL-5 was identified as a key cytokine in NP tissue, and its presence was demonstrated in eosinophils and associated with eosinophilia (26). In addition to their activation and survival, eosinophil recruitment into nasal tissue is another key process in the induction of eosinophilia. Chemokines are a large group of proteins that participate in the recruitment of immune cells into tissue by binding G protein-coupled receptors on their target cells. There are three known human eotaxin chemokines, namely, eotaxin-1 (CCL11), eotaxin-2 (CCL24), and eotaxin-3 (CCL26) (27). It has been reported that levels of each of these proteins are increased in patients with eosinophilic CRSwNP compared to healthy controls. Furthermore, several investigators have established a correlation between eotaxins and tissue eosinophilia in CRSwNP (2829). These results indicate that eotaxins are key chemotactic factors acting on eosinophils in this condition. The effect of hypoxia and anoxia on NP-derived fibroblasts has been demonstrated previously. In these cells, hypoxia results in increases in vascular endothelial growth factor, TGF-β, IL-8, and eotaxin-1, the latter being involved in eosinophil recruitment (30). Indeed, chemokines not only contribute to the orchestration of cellular interactions in CRS, but are also key biomarkers of the diverse endotypes of this disease.
Moreover, it has recently been reported that hypoxia modulates human eosinophil function. However, to the best of our knowledge, no study has been performed to test the existence of a direct link between eosinophils and hypoxia in CRS (31). The mechanism underlying enhanced eosinophil survival in hypoxic microenvironments needs to be further explored.
Mast cells have also been examined in the context of CRS because of their ability to recruit eosinophils and basophils and produce biological mediators that can induce vasodilation and tissue edema. Takabayashi et al. found that mucosal epithelial mast cells have a tryptase phenotype (32). Cao et al . also reported significantly increased numbers of tryptase-positive mast cells in the epithelia of eosinophilic NPs in Chinese patients (33). These recent findings indicate that tryptase-positive mast cell presence is highly elevated in the epithelia of individuals with eosinophilic CRSwNP. However, the specific role of the epithelial layer in the direct activation of mast cells remains unclear. It may be hypothesized that epithelial cells produce a combination of cytokines in response to different stimuli that directly induces mast cells and other immune cells to in turn release Th2 cytokines (22).
Furthermore, the number of basophils, which often have a close relationship with mast cells, has recently been shown to be increased in NP tissue (34). Thus, the increased presence of eosinophils, basophils, and mast cells in nasal mucosal tissue suggests that these cells are implicated in sinus disease pathogenesis. Treatment that eliminates the recruitment and activation of immune cells might therefore be expected to exert beneficial effects for patients with CRS.
The respiratory epithelium constitutes the barrier between external and internal environments and is crucial for the protection of the sinonasal mucosal interior milieu. The most basic function of the epithelium relies on its ability to form tight junctions between cells, creating a physical barrier between the airway lumen and subepithelial tissue. Tight junction-associated proteins, such as zona occludens-1, junctional adhesion molecule-A, cingulin, occludin, and claudins, are central to epithelial cytoprotection at the cellular level. Decreased epithelial cohesion and integrity can allow entrance of pathogens and environmental antigens into the sinonasal mucosa. Several studies have examined sinus mucosal function and integrity in patients with CRS (3536).
Sinonasal mucosal inflammation and swelling cause blockage of the sinus ostia, compromising the normal ventilation of the sinuses. Thus, such processes may generate an environment of reduced oxygen tension. In 2012, Shin et al . (37) reported that hypoxia-inducible factor (HIF)-1 mediates nasal polypogenesis by inducing EMT. HIF activation was observed in primary human epithelial and RPMI 2650 cells exposed to hypoxia (1% oxygen tension), as a result of which, all cells underwent EMT. EMT is a process in which epithelial cells lose their epithelial character and develop mesenchymal properties. During EMT, the epithelial marker protein E-cadherin is down-regulated, while the mesenchymal markers N-cadherin and Snail are up-regulated. Recently, Hupin et al . also documented features of EMT in CRSsNP and CRSwNP patients (38). In more recent work, Lee et al . (39) investigated the role of sirtuin 1 (SIRT1) in NP formation using an animal model of CRS. SIRT1 was found to fine-tune cellular responses to hypoxia by deacetylating HIF-1α and HIF-2α (4041). In addition, resveratrol (a SIRT1 activator) treatment suppressed nasal polypogenesis in C57BL/6 mice (42). According to these results, HIF-1 inhibitors and SIRT1 might represent therapeutic targets for the treatment of nasal polyposis due to their inhibition of HIF-1-induced tissue remodeling (37).
There is considerable evidence of epithelial cell activation in patients with CRS. Although not traditionally considered immune cells, epithelial cells play a critical role in the innate immune response (43). The cytokines IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) are produced and released by nasal epithelial cells in response to various stimuli or cellular damage. These epithelial-derived cytokines can affect both innate and adaptive type 2 immunity and cause remodeling of and pathological changes in the upper airways, suggesting pivotal roles in the pathophysiology of CRS.
The IL-17 cytokine family includes six members, which exhibit pro- and anti-inflammatory activities (4445464748). Of these, IL-17E (also known as IL-25) has recently drawn much attention (49). Intraperitoneal or intranasal administration of IL-25 results in the presence of eosinophils and Th2 cytokines in bronchoalveolar lavage fluid and lung tissue in vivo (505152). Moreover, blocking IL-25 decreases eosinophil accumulation and Th2 cytokine production in a murine model of NPs and asthma (5354). Lam et al . reported that IL-25 mRNA levels are significantly elevated in ethmoid sinuses in CRSwNP compared to CRSsNP and controls. Similarly, IL-25 mRNA and protein expression is increased in NP mucosa in Korean patients. Furthermore, IL-17 receptor levels are heightened in the immune cells of patients with NPs compared to control subjects, indicating that IL-25 may contribute to tissue remodeling and CRSwNP pathogenesis. (54). In contrast, Miljkovic et al. reported IL-25 mRNA levels to be significantly decreased in NPs compared to the ethmoid sinuses of control subjects and CRSsNP patients (55).
IL-33, a member of the IL-1 superfamily of cytokines, is expressed by epithelial cells and is up-regulated in response to pro-inflammatory stimulation. The IL-33 protein localizes to the nucleus in producing cells, and there is no evidence to suggest its cytoplasmic localization. Recently, induction of IL-33 has been observed in epithelial cells from patients with CRS (56). IL-33 receptor is expressed on memory Th2 cells, mast cells, basophils, and NK T cells (5758). It has been reported that IL-33 mRNA is highly expressed in nasal mucosa but its levels are not elevated in NPs (5559). More recently, Kim et al. described increased expression of IL-33 mRNA and protein in patients with CRSwNP. Interestingly, the concentration of IL-33 protein in CRSwNP positively correlates with neutrophil number and the expression of several Th1 and Th17 inflammatory markers (60).
Like IL-33, TSLP is a cytokine most abundantly expressed by cells of the epithelium. Nagarkar et al. and Allakhverdi et al . found TSLP activity to be higher in NPs than in control tissue (1461). Moreover, prolonged allergen exposure has been associated with upregulation of TSLP in an ovalbumin/staphylococcal enterotoxin B-induced CRS model (62). Given these findings, further work is needed to extensively characterize the cytokine environment in CRSsNP, as well as CRSwNP patients. Thus, the upper airway epithelium can no longer be regarded merely as a structural barrier, but should be considered an active player in the pathogenesis of CRS.
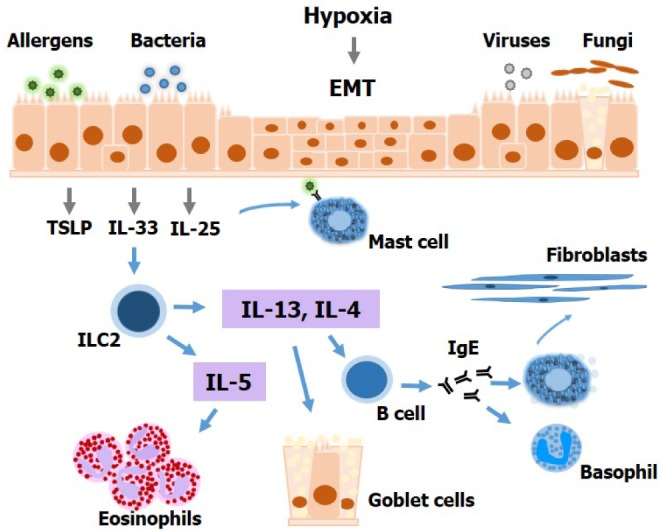
As described above, IL-25, IL-33, and TSLP represent a novel array of epithelial pro-inflammatory cytokines that promote Th2 inflammation in the upper airways and could serve as therapeutic targets in CRSwNP. The epithelial barrier alterations and epithelial-derived inflammatory cytokines that drive innate Th2 inflammation have not been fully investigated in the clinical context of CRS (Fig. 1). Recently, preclinical models, mouse and rat models in particular, have been widely used to understand the mechanisms underlying human diseases. As experimental allergens, most research groups have utilized and confirmed the efficacy of ovalbumin and house dust mite extract in combination with Staphylococcus aureus enterotoxin B to induce eosinophilic CRS with nasal polypoid lesions. Therefore, further evaluation of candidate therapies for the treatment of CRS could be accelerated using animal models of CRS and NPs (636465).
CRS is a complex inflammatory condition and many factors contribute to its development. Although recent research efforts have expanded our understanding of its pathogenesis, further investigations of immune cell responses and epithelial function in normal and pathologic sinonasal mucosae are necessary. In addition, the preclinical and clinical application of such knowledge of the immunologic changes taking place in CRS could facilitate the development of novel therapeutic strategies to improve its treatment.
ACKNOWLEDGEMENTS
This work was supported by Creative-Pioneering Researchers Program through Seoul National University (SNU) and the Education and Research Encouragement Fund of Seoul National University Hospital (2017).
References
1. Hamilos DL. Chronic rhinosinusitis: epidemiology and medical management. J Allergy Clin Immunol. 2011; 128:693–707. PMID: 21890184.

2. Soler ZM, Mace JC, Litvack JR, Smith TL. Chronic rhinosinusitis, race, and ethnicity. Am J Rhinol Allergy. 2012; 26:110–116. PMID: 22487286.

3. Stevens WW, Lee RJ, Schleimer RP, Cohen NA. Chronic rhinosinusitis pathogenesis. J Allergy Clin Immunol. 2015; 136:1442–1453. PMID: 26654193.

4. Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi-Peters A, Grammer LC, Schleimer RP. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol. 2008; 22:549–559. PMID: 18786300.

5. Payne SC, Borish L, Steinke JW. Genetics and phenotyping in chronic sinusitis. J Allergy Clin Immunol. 2011; 128:710–720. PMID: 21704364.

6. Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH, Wang DY, Desrosiers M, Liu Z. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009; 124:478–484. 484.e1–484.e2. PMID: 19541359.

7. Kim JW, Hong SL, Kim YK, Lee CH, Min YG, Rhee CS. Histological and immunological features of non-eosinophilic nasal polyps. Otolaryngol Head Neck Surg. 2007; 137:925–930. PMID: 18036422.

8. Ikeda K, Shiozawa A, Ono N, Kusunoki T, Hirotsu M, Homma H, Saitoh T, Murata J. Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope. 2013; 123:E1–E9. PMID: 23670893.

9. Wen W, Liu W, Zhang L, Bai J, Fan Y, Xia W, Luo Q, Zheng J, Wang H, Li Z, Xia J, Jiang H, Liu Z, Shi J, Li H, Xu G. Nasal Health Group, China (NHGC). Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol. 2012; 129:1522–1528. PMID: 22460066.

10. Kim SJ, Lee KH, Kim SW, Cho JS, Park YK, Shin SY. Changes in histological features of nasal polyps in a Korean population over a 17-year period. Otolaryngol Head Neck Surg. 2013; 149:431–437. PMID: 23812744.

11. Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, Bachert C. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006; 61:1280–1289. PMID: 17002703.

12. Van Bruaene N, Perez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, Schmidt-Weber C, Akdis C, Van Cauwenberge P, Bachert C, Gevaert P. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008; 121:1435–1441. PMID: 18423831.

13. Park SJ, Kim TH, Jun YJ, Lee SH, Ryu HY, Jung KJ, Jung JY, Hwang GH, Lee SH. Chronic rhinosinusitis with polyps and without polyps is associated with increased expression of suppressors of cytokine signaling 1 and 3. J Allergy Clin Immunol. 2013; 131:772–780. PMID: 23375208.

14. Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, Suh LA, Norton J, Harris KE, Grammer LC, Chandra RK, Conley DB, Kern RC, Schleimer RP, Kato A. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013; 132:593–600. PMID: 23688414.

15. Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, Van Cauwenberge P, Bachert C. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008; 122:961–968. PMID: 18804271.

16. Van Bruaene N, Derycke L, Perez-Novo CA, Gevaert P, Holtappels G, De Ruyck N, Cuvelier C, Van Cauwenberge P, Bachert C. TGF-beta signaling and collagen deposition in chronic rhinosinusitis. J Allergy Clin Immunol. 2009; 124:253–259. PMID: 19500825.
17. Sejima T, Holtappels G, Kikuchi H, Imayoshi S, Ichimura K, Bachert C. Cytokine profiles in Japanese patients with chronic rhinosinusitis. Allergol Int. 2012; 61:115–122. PMID: 22377524.

18. Shi LL, Xiong P, Zhang L, Cao PP, Liao B, Lu X, Cui YH, Liu Z. Features of airway remodeling in different types of Chinese chronic rhinosinusitis are associated with inflammation patterns. Allergy. 2013; 68:101–109. PMID: 23157215.

19. Van Bruaene N, C PN, Van Crombruggen K, De Ruyck N, Holtappels G, Van Cauwenberge P, Gevaert P, Bachert C. Inflammation and remodelling patterns in early stage chronic rhinosinusitis. Clin Exp Allergy. 2012; 42:883–890. PMID: 22093003.

20. Bachert C, Van Bruaene N, Toskala E, Zhang N, Olze H, Scadding G, Van Drunen CM, Mullol J, Cardell L, Gevaert P, Van Zele T, Claeys S, Hallden C, Kostamo K, Foerster U, Kowalski M, Bieniek K, Olszewska-Ziaber A, Nizankowska-Mogilnicka E, Szczeklik A, Swierczynska M, Arcimowicz M, Lund V, Fokkens W, Zuberbier T, Akdis C, Canonica G, Van Cauwenberge P, Burney P, Bousquet J. Important research questions in allergy and related diseases: 3-chronic rhinosinusitis and nasal polyposis - a GALEN study. Allergy. 2009; 64:520–533. PMID: 19317839.
21. Polzehl D, Moeller P, Riechelmann H, Perner S. Distinct features of chronic rhinosinusitis with and without nasal polyps. Allergy. 2006; 61:1275–1279. PMID: 17002702.

22. Kato A. Immunopathology of chronic rhinosinusitis. Allergol Int. 2015; 64:121–130. PMID: 25838086.

23. Katotomichelakis M, Tantilipikorn P, Holtappels G, De Ruyck N, Feng L, Van Zele T, Muangsomboon S, Jareonchasri P, Bunnag C, Danielides V, Cuvelier CA, Hellings PW, Bachert C, Zhang N. Inflammatory patterns in upper airway disease in the same geographical area may change over time. Am J Rhinol Allergy. 2013; 27:354–360. PMID: 23816657.

24. Park YM, Bochner BS. Eosinophil survival and apoptosis in health and disease. Allergy Asthma Immunol Res. 2010; 2:87–101. PMID: 20358022.

25. Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009; 21:1303–1309. PMID: 19819937.

26. Bachert C, Wagenmann M, Hauser U, Rudack C. IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol. 1997; 99:837–842. PMID: 9215253.
27. Mackay CR. Chemokines: immunology's high impact factors. Nat Immunol. 2001; 2:95–101. PMID: 11175800.

28. Yao T, Kojima Y, Koyanagi A, Yokoi H, Saito T, Kawano K, Furukawa M, Kusunoki T, Ikeda K. Eotaxin-1, -2, and -3 immunoreactivity and protein concentration in the nasal polyps of eosinophilic chronic rhinosinusitis patients. Laryngoscope. 2009; 119:1053–1059. PMID: 19296494.

29. Olze H, Forster U, Zuberbier T, Morawietz L, Luger EO. Eosinophilic nasal polyps are a rich source of eotaxin, eotaxin-2 and eotaxin-3. Rhinology. 2006; 44:145–150. PMID: 16792175.
30. Early SB, Hise K, Han JK, Borish L, Steinke JW. Hypoxia stimulates inflammatory and fibrotic responses from nasal-polyp derived fibroblasts. Laryngoscope. 2007; 117:511–515. PMID: 17334314.

31. Nissim Ben Efraim AH, Eliashar R, Levi-Schaffer F. Hypoxia modulates human eosinophil function. Clin Mol Allergy. 2010; 8:10. PMID: 20642833.

32. Takabayashi T, Kato A, Peters AT, Suh LA, Carter R, Norton J, Grammer LC, Tan BK, Chandra RK, Conley DB, Kern RC, Fujieda S, Schleimer RP. Glandular mast cells with distinct phenotype are highly elevated in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012; 130:410–420. PMID: 22534535.

33. Cao PP, Zhang YN, Liao B, Ma J, Wang BF, Wang H, Zeng M, Liu WH, Schleimer RP, Liu Z. Increased local IgE production induced by common aeroallergens and phenotypic alteration of mast cells in Chinese eosinophilic, but not non-eosinophilic, chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2014; 44:690–700. PMID: 24597471.

34. Mahdavinia M, Carter RG, Ocampo CJ, Stevens W, Kato A, Tan BK, Kern RC, Conley DB, Chandra R, Hulse KE, Suh LA, Norton JE, Peters AT, Grammer LC, Schwartz LB, Schleimer RP. Basophils are elevated in nasal polyps of patients with chronic rhinosinusitis without aspirin sensitivity. J Allergy Clin Immunol. 2014; 133:1759–1763. PMID: 24636088.

35. Soyka MB, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, Wanke K, Kast JI, Akdis CA. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-gamma and IL-4. J Allergy Clin Immunol. 2012; 130:1087–1096. PMID: 22840853.
36. Nomura K, Obata K, Keira T, Miyata R, Hirakawa S, Takano K, Kohno T, Sawada N, Himi T, Kojima T. Pseudomonas aeruginosa elastase causes transient disruption of tight junctions and downregulation of PAR-2 in human nasal epithelial cells. Respir Res. 2014; 15:21. PMID: 24548792.

37. Shin HW, Cho K, Kim DW, Han DH, Khalmuratova R, Kim SW, Jeon SY, Min YG, Lee CH, Rhee CS, Park JW. Hypoxia-inducible factor 1 mediates nasal polypogenesis by inducing epithelial-to-mesenchymal transition. Am J Respir Crit Care Med. 2012; 185:944–954. PMID: 22323302.

38. Hupin C, Gohy S, Bouzin C, Lecocq M, Polette M, Pilette C. Features of mesenchymal transition in the airway epithelium from chronic rhinosinusitis. Allergy. 2014; 69:1540–1549. PMID: 25104359.

39. Lee M, Kim DW, Yoon H, So D, Khalmuratova R, Rhee CS, Park JW, Shin HW. Sirtuin 1 attenuates nasal polypogenesis by suppressing epithelial-to-mesenchymal transition. J Allergy Clin Immunol. 2016; 137:87–98. PMID: 26342525.

40. Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell. 2010; 38:864–878. PMID: 20620956.
41. Yoon H, Shin SH, Shin DH, Chun YS, Park JW. Differential roles of Sirt1 in HIF-1alpha and HIF-2alpha mediated hypoxic responses. Biochem Biophys Res Commun. 2014; 444:36–43. PMID: 24423936.
42. Kim SW, Kim DW, Khalmuratova R, Kim JH, Jung MH, Chang DY, Shin EC, Lee HK, Shin HW, Rhee CS, Jeon SY, Min YG. Resveratrol prevents development of eosinophilic rhinosinusitis with nasal polyps in a mouse model. Allergy. 2013; 68:862–869. PMID: 23751068.

43. Zhang N, Van Crombruggen K, Gevaert E, Bachert C. Barrier function of the nasal mucosa in health and type-2 biased airway diseases. Allergy. 2016; 71:295–307. PMID: 26606240.

44. Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, O'Connor W Jr, Rongvaux A, Van Rooijen N, Haberman AM, Iwakura Y, Kuchroo VK, Kolls JK, Bluestone JA, Herold KC, Flavell RA. Control of TH17 cells occurs in the small intestine. Nature. 2011; 475:514–518. PMID: 21765430.

45. Ke Y, Liu K, Huang GQ, Cui Y, Kaplan HJ, Shao H, Sun D. Anti-inflammatory role of IL-17 in experimental autoimmune uveitis. J Immunol. 2009; 182:3183–3190. PMID: 19234216.

46. Otani K, Watanabe T, Tanigawa T, Okazaki H, Yamagami H, Watanabe K, Tominaga K, Fujiwara Y, Oshitani N, Arakawa T. Anti-inflammatory effects of IL-17A on Helicobacter pylori-induced gastritis. Biochem Biophys Res Commun. 2009; 382:252–258. PMID: 19249291.

47. Pappu R, Ramirez-Carrozzi V, Sambandam A. The interleukin-17 cytokine family: critical players in host defence and inflammatory diseases. Immunology. 2011; 134:8–16. PMID: 21726218.

48. Zizzo G, Cohen PL. IL-17 stimulates differentiation of human anti-inflammatory macrophages and phagocytosis of apoptotic neutrophils in response to IL-10 and glucocorticoids. J Immunol. 2013; 190:5237–5246. PMID: 23596310.

49. Lee M, Kim DW, Shin HW. Targeting IL-25 as a novel therapy in chronic rhinosinusitis with nasal polyps. Curr Opin Allergy Clin Immunol. 2017; 17:17–22. PMID: 27870664.

50. Pappu R, Rutz S, Ouyang W. Regulation of epithelial immunity by IL-17 family cytokines. Trends Immunol. 2012; 33:343–349. PMID: 22476048.

51. Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G, Coffman RL. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002; 169:443–453. PMID: 12077275.

52. Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001; 15:985–995. PMID: 11754819.
53. Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, Sturton G, Wong SH, McKenzie AN. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007; 120:1324–1331. PMID: 17889290.

54. Shin HW, Kim DK, Park MH, Eun KM, Lee M, So D, Kong IG, Mo JH, Yang MS, Jin HR, Park JW, Kim DW. IL-25 as a novel therapeutic target in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2015; 135:1476–1485. PMID: 25725991.

55. Miljkovic D, Bassiouni A, Cooksley C, Ou J, Hauben E, Wormald PJ, Vreugde S. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy. 2014; 69:1154–1161. PMID: 24924975.

56. Mjosberg JM, Trifari S, Crellin NK, Peters CP, Van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011; 12:1055–1062. PMID: 21909091.

57. Smith DE. IL-33: a tissue derived cytokine pathway involved in allergic inflammation and asthma. Clin Exp Allergy. 2010; 40:200–208. PMID: 19906013.

58. Bulek K, Swaidani S, Aronica M, Li X. Epithelium: the interplay between innate and Th2 immunity. Immunol Cell Biol. 2010; 88:257–268. PMID: 20065993.

59. Lam M, Hull L, McLachlan R, Snidvongs K, Chin D, Pratt E, Kalish L, Sacks R, Earls P, Sewell W, Harvey RJ. Clinical severity and epithelial endotypes in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013; 3:121–128. PMID: 23038685.

60. Kim DK, Jin HR, Eun KM, Mo JH, Cho SH, Oh S, Cho D, Kim DW. The role of interleukin-33 in chronic rhinosinusitis. Thorax. 2016; DOI: 10.1136/thoraxjnl-2016-208772.

61. Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M, Liu YJ, Howie KJ, Denburg JA, Gauvreau GM, Delespesse G. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol. 2009; 123:472–478. PMID: 19064280.
62. Kim DW, Eun KM, Jin HR, Cho SH, Kim DK. Prolonged allergen exposure is associated with increased thymic stromal lymphopoietin expression and Th2-skewing in mouse models of chronic rhinosinusitis. Laryngoscope. 2016; 126:E265–E272. PMID: 27107152.

63. Kim DW, Khalmuratova R, Hur DG, Jeon SY, Kim SW, Shin HW, Lee CH, Rhee CS. Staphylococcus aureus enterotoxin B contributes to induction of nasal polypoid lesions in an allergic rhinosinusitis murine model. Am J Rhinol Allergy. 2011; 25:e255–e261. PMID: 22185735.

64. Khalmuratova R, Lee M, Kim DW, Park JW, Shin HW. Induction of nasal polyps using house dust mite and Staphylococcal enterotoxin B in C57BL/6 mice. Allergol Immunopathol(Madr.). 2016; 44:66–75. PMID: 26242569.

Figure 1
Altered epithelial barrier function contributes to type 2-mediated inflammation in chronic rhinosinusitis and nasal polyps. Several factors, including allergens, bacteria, viruses, and fungi can activate nasal epithelial cells to produce innate cytokines that activate type 2 innate lymphoid cells. IL-5 derived from these latter contributes to airway eosinophilia, whereas IL-13 acts directly on the epithelium to drive goblet cell metaplasia. IL-4 promotes IgE production by B cells, which causes mast cell and basophil activation. Mast cells activate fibroblasts to produce collagen fibers. In addition, mast cells produce mediators that can induce vasodilation and tissue edema. Nasal epithelial cells can also undergo epithelial-to-mesenchymal transition under hypoxia, as observed in chronic rhinosinusitis and nasal polyps. EMT, epithelial-to-mesenchymal transition; ILC2, type 2 innate lymphoid cell.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download