Abstract
Ambroxol is used in COPD and asthma to increase mucociliary clearance and regulate surfactant levels, perhaps through anti-oxidant and anti-inflammatory activities. To determine the role and effect of ambroxol in an experimental model of asthma, BALB/c mice were sensitized to ovalbumin (OVA) followed by 3 days of challenge. Airway hyperresponsiveness (AHR), lung cell composition and histology, and cytokine and protein carbonyl levels in bronchoalveolar lavage (BAL) fluid were determined. Ambroxol was administered either before the first OVA challenge or was begun after the last allergen challenge. Cytokine production levels from lung mononuclear cells (Lung MNCs) or alveolar macrophages (AM) were also determined. Administration of ambroxol prior to challenge suppressed AHR, airway eosinophilia, goblet cell metaplasia, and reduced inflammation in subepithelial regions. When given after challenge, AHR was suppressed but without effects on eosinophil numbers. Levels of IL-5 and IL-13 in BAL fluid were decreased when the drug was given prior to challenge; when given after challenge, increased levels of IL-10 and IL-12 were detected. Decreased levels of protein carbonyls were detected in BAL fluid following ambroxol treatment after challenge. In vitro, ambroxol increased levels of IL-10, IFN-γ, and IL-12 from Lung MNCs and AM, whereas IL-4, IL-5, and IL-13 production was not altered. Taken together, ambroxol was effective in preventing AHR and airway inflammation through upregulation of Th1 cytokines and protection from oxidative stress in the airways.
Asthma is the most prevalent chronic respiratory disease and the prevalence is still increasing (1). Outcomes of asthma treatments such as mortality rates were improved, but have stalled in the last decade (2). Excessive mucus production is a major component of many respiratory diseases, including bronchial asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis (3).
In asthma, airway mucus hypersecretion is thought to be a pathophysiological feature and contributor to morbidity and mortality (4). In particular, IL-13 was shown to be a potent inducer of airway hyperresponsiveness (AHR), eosinophilic airway inflammation, and goblet cell metaplasia (5). Upregulation of MUC5AC, a mucin-inducing gene, was related to disease severity in asthmatics and in animal models (67). IL-13 has been shown to be a major inducer of mucus production through goblet cell metaplasia in airway epithelial layers and to play a critical role in the development of AHR (8). Recent studies revealed that the epidermal growth factor receptor (EGF-R) appeared to be a convergent pathway for a number of mediator signals; EGF-R was upregulated in the airways of asthmatic patients (9). These signaling molecules have been studied as potential targets of pharmacotherapy to inhibit airway mucus production in asthma (8). On the other hand, appropriate levels of mucus production are essential for protection from airborne pathogens or allergens and probably for the clearance of inflammatory chemicals or dead cells. However, precise mechanisms and effects on mucus production and secretion in the pathogenesis of asthma are unclear.
Accordingly, expectorants such as ambroxol have been widely used in these mucus-related respiratory diseases (101112). Ambroxol is a metabolite of bromhexine, which has been used in the therapy of airway diseases since the late 1970s (13). In asthmatics, ambroxol was shown to improve AHR and lung function (1415). Ambroxol has also shown anti-oxidant effects through upregulation of thioredoxin and/or thioredoxin reductase, and inhibited oxygen free radical production (1216). Ambroxol also maintains the airway surface environment by modulating surfactant protein levels in the airways and increased mucociliary clearance (1718). Based on these results, clinical trials with ambroxol to prevent or treat infant respiratory distress syndrome have been carried out (1920). In a mouse model of lipopolysaccharide-induced acute lung injury, ambroxol treatment reduced lung inflammation and damage as well as cytokine levels in bronchoalveolar lavage (BAL) fluid, including IL-6, TNF-α, and TGF-β (21). In basophils, ambroxol inhibited secretion of IL-4, IL-13, and histamine (22). However, the underlying mechanisms whereby ambroxol exhibits anti-inflammatory effects in the airways such as in asthma remain to be defined.
The socioeconomic burden in the treatment of asthma has been of worldwide concern with increasing prevalence of the disease (12). Safe and cost-effective therapeutic alternatives are needed. Ambroxol has been in the clinic for many years, the drug is inexpensive and few adverse effects have been described. Therefore, understanding the potential of the drug in the treatment of asthma is a valid concern. In this study, ambroxol was administrated to mice in a model of allergen-induced AHR and airway inflammation. The drug was administered prior to and after allergen challenge and changes in airway inflammation and AHR were monitored.
Female BALB/c mice, 6-8 weeks of age, were obtained from Jackson Laboratories (Bar Harbor, ME, USA). The animals were maintained on an ovalbumin (OVA)-free diet. Experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee of the National Jewish Health.
Mice were sensitized by intraperitoneal (i.p.) injection of 20 µg of OVA (Grade V, Sigma Chemical Co., St. Louis, MO, USA) emulsified in 2.0 mg alum (AlumImuject: Pierce, Rockford, IL, USA) in a total volume of 100 µl on days 1 and 14. Mice were challenged via the airways with OVA (1% in saline) for 20 min on days 28, 29, and 30 by ultrasonic nebulizer (model NE-U07, Omron Healthcare, Vernon Hills, IL, USA). Control mice received PBS as sham-sensitization followed by OVA aerosol challenges in the same fashion. To determine the effects of ambroxol on airway allergic inflammation and AHR, 10 mg/kg of ambroxol (Sigma) in 200 µl saline or saline alone as a vehicle control were administered twice a day i.p. after sensitization and beginning 2 days before through the 3 days of allergen challenges (Fig. 1A). In other experiments, as illustrated in Fig. 1B, ambroxol treatments were initiated after the last challenge and completed prior to the last day of study. The dose of ambroxol was chosen based on previous studies (2123) and preliminary studies.
Airway responsiveness was assessed as changes in airway function after challenge with aerosolized methacholine (MCh) (Sigma). Mice were anesthetized, tracheostomized, mechanically ventilated, and lung function was assessed as described previously (24). Ventilation was achieved at 160 breaths per minute at a tidal volume of 0.16 ml with a positive end-expiratory pressure of 2-4 cm H2O. Lung resistance (RL) was continuously computed (Labview, National Instruments, Austin, TX, USA) by fitting flow, volume, and pressure to an equation of motion, using a recessive least squares algorithm.
Aerosolized MCh was administered through bypass tubing via an ultrasonic nebulizer (model 5500D, DeVilbiss, Somerset, PA, USA) placed between the expiratory port of the ventilator and the four-way connector. Aerosolized MCh was administered for 8 seconds with a tidal volume of 0.45 ml and a frequency of 60 BPM using another ventilator (model 683, Harvard Apparatus, South Natick, MA, USA). The data for RL were continuously collected for up to 3 minutes and maximum values were taken.
Immediately after assessment of AHR, lungs were lavaged via the tracheal tube with Hank's balanced solution (HBSS, 1×1 ml 37℃). The volume of collected BAL fluid was measured in each sample and the number of leukocytes was counted (Coulter Counter, Coulter Corporation, Hialeah, FL, USA). Differential cell counts were performed by counting at least 200 cells on cytocentrifuged preparations (Shandon Cytosipin3, ThermoFisher Scientific, Waltham, MA). Slides were stained with modified Wright-Giemsa and white blood cells were differentiated by standard hematological procedures in a blinded fashion. BAL fluid supernatants were collected and stored at –70℃ until measurements were performed.
To determine the effects of ambroxol In vitro, lung mononuclear cells (MNCs) were isolated and cultured with OVA and ambroxol under various conditions. Briefly, lungs were taken from mice following exposure to OVA. Cells in BAL fluid were collected from mice following sensitization with 1 ml of HBSS four times. Over 99% of cells in the BAL fluid obtained in this way after sensitization but not challenge were macrophages. Lung MNCs were obtained following collagenase digestion of the lung followed by 35% Percoll (Sigma) gradient centrifugation. Two hundred thousand lung MNCs or 1×105 alveolar macrophages collected from BAL were cultured with or without 10 µg/ml of OVA. Ambroxol was added to these cultures at concentrations of 0.01, 0.1, 1, and 10 µM. After 24 hrs, supernatants were collected and stored for cytokine and protein carbonyl measurements as described below.
Cytokine levels in the BAL fluid or supernatants from cultured cells were measured as previously described (25). Briefly, measurements of IL-4, IL-5, IL-10, and IL-12 were performed by ELISA (BD PharMingen, San Diego, CA, USA) with 96-well plates (Immulon 2; Dynatech, Chantilly, VA, USA); IL-13 measurements were performed using an ELISA kit (QuantikineM; R&D Systems, Minneapolis, MN, USA) for BAL fluid and ELISA set (DuoSet; R&D Systems) for In vitro cell culture supernatants, all following the manufacturer's protocol. The limits of detection were 1.5 pg/ml for IL-13, 4 pg/ml for IL-4 and IL-5, 10 pg/ml for IL-10 and IFN-γ, and 62.5 pg/ml for IL-13 ELISA set.
To investigate the role of ambroxol as an anti-oxidant agent, we determined protein carbonyl levels in BAL fluid as an index of oxidative stress in the lung (26). The levels of protein carbonyls in BAL fluid were measured by ELISA (Zentec PC kit; Zenith Technology Corporation, Dunedin, New Zealand).
After BAL fluid was collected, lungs were inflated with 1 ml 10% formalin through the trachea, and fixed in formalin by immersion. Tissue blocks of lung tissue from 4-5 mice in each group were cut from around the main bronchus and embedded in paraffin blocks; 2-3 tissue sections (5-µm) per mouse were then affixed to microscope slides and deparaffinized. The slides were then stained with hematoxylin and eosin (H/E) and periodic acid-Schiff (PAS). The slides were taken to pictures with a microscope (BX40; Olympus America Inc., Melville, NY, USA) equipped with a digital camera (Q-color 3; Olympus America Inc.) and images were stored on a Macintosh computer. Goblet cell metaplasia was quantified as the number of pixels on the computer converted from PAS+ areas along the airway epithelium. The quantification was performed using NIH ImageJ software (version 1.38), available on the internet at http://rsb.info.nih.gov/ij/ download. html. Four different fields per slide in 8 samples from each group of mice were examined in a blinded manner.
All results were expressed as the mean±standard error of the mean (SEM). The Tukey-Kramer test was used for comparisons between multiple groups. Since measured values may not be normally distributed on account of the small sample sizes, nonparametric analysis, Mann-Whitney U test, was also used to confirm that the statistical differences remained significant. The p values for significance were set to 0.05 for all tests.
Mice sensitized to OVA followed by OVA challenge developed AHR in response to inhaled MCh (Fig. 2A) and increased numbers of eosinophils in the BAL fluid (Fig. 2B) compared to sham-sensitized mice. When ambroxol treatment was initiated prior to allergen challenge in OVA-sensitized mice, the numbers of eosinophils were decreased by approximately 50% and airway responsiveness to MCh was also decreased significantly compared to vehicle-treated mice. Ambroxol treatment of sham-sensitized mice did not show any effects.
As shown in Fig. 1C, BAL fluid was obtained following airway function measurements and cytokine levels were determined. The levels of IL-4, IL-5, IL-13, and IL-12 were significantly increased in the BAL fluid from OVA-sensitized and -challenged mice compared to shamsensitized mice. In the mice treated with ambroxol prior to OVA challenge, IL-5 and IL-13 levels were decreased.
Histological analysis revealed that inflammatory cell infiltration into subepithelial areas and increased numbers of PAS+ goblet cells could be demonstrated in the airways of OVA-sensitized and challenged mice (Fig. 2D and 2E). In mice which received ambroxol prior to the OVA challenges, reduced levels of both inflammatory cells and PAS-staining goblet cell metaplasia were observed in lung tissue sections.
Levels of protein carbonyls in BAL fluid were not altered by ambroxol treatment initiated prior to the allergen challenge (data not shown).
When ambroxol treatment was initiated following OVA challenge of sensitized mice, the development of AHR was significantly decreased compared to the control group (Fig. 3A). However, ambroxol treatment initiated after completion of the OVA challenges showed no effect on the number of eosinophils in the BAL fluid (Fig. 3B).
As shown in Fig. 3C, cytokine analysis of BAL fluid showed that levels of IL-10, IFN-γ and IL-12 were significantly increased with ambroxol treatment initiated following OVA challenge compared to vehicle-treated controls. However, the levels of IL-4, IL-5, and IL-13 were not altered with ambroxol treatment. Ambroxol treatment in sham-sensitized mice showed no effect on cytokine levels or cell composition in BAL fluid or AHR.
In contrast to ambroxol treatment begun prior to OVA challenge, histological analysis revealed that ambroxol treatment initiated following OVA challenge did not alter inflammatory cell infiltration or goblet cell metaplasia in the airways of OVA-sensitized and challenged mice (Fig. 3D and 3E).
Levels of protein carbonyls in BAL fluid were assayed to determine the degree of oxidative damage in the airways. As shown in Fig. 3F, protein carbonyl levels were increased in the BAL fluid from mice that were sensitized and challenged to OVA compared to the shamsensitized mice. These levels were decreased when ambroxol treatment was initiated after the completion of OVA challenge compared to vehicle-treated mice.
Lung MNCs were collected and purified from mice after sensitization and challenge to OVA and cytokine levels in the supernatants of cultured cells were determined. The In vitro production of Th2-type cytokines, IL-4, IL-5, and IL-13 was increased from cells stimulated with OVA and these levels were not altered by inclusion of ambroxol in the cultures (Fig. 4A). IL-10 was increased following addition of OVA and was increased further in the presence of ambroxol in a dose-dependent manner. IFN-γ and IL-12 secretion levels were not altered with OVA, but increased with ambroxol in a dose-dependent manner.
Cytokine secretion levels from alveolar macrophages were analyzed following culture with OVA and ambroxol (Fig. 4B). The levels of IL-10, IFN-γ and IL-12 levels were not altered by OVA, but were increased on addition of ambroxol in a dose-dependent manner. Th2 cytokines, IL-4, IL-5, and IL-13 were not detected in the macrophage cultures (data not shown).
Ambroxol has been widely used since the late 1970s in various respiratory diseases with the goal of improving mucus clearance, facilitating expectoration, easing of productive cough, and providing local anesthetic effects (1327). Ambroxol has been used to treat asthmatics (28). The data, albeit limited, suggest some effects in improving airway responsiveness and inflammation (14). However, the mechanisms underlying these effects are not well understood. In this study, OVAsensitized and -challenged mice developed AHR and eosinophilic airway inflammation, accompanied by increased levels of the Th2 cytokines, IL-4, IL-5, and IL-13. When ambroxol treatment was initiated prior to OVA challenge of sensitized mice, decreased levels of airway responsiveness, eosinophilia, and goblet cell metaplasia along the airways were observed, accompanied by decreases in the levels of the Th2-type cytokines, IL-5, and IL-13 in BAL fluid. Asthma is a complex disease in which numerous factors contribute to the characteristic features of the disease, persistent airway dysfunction including AHR and allergic airway inflammation (29). Among these factors, Th2 cells and related cytokines, IL-4, IL-5, and IL-13 are thought to play a central role in the pathogenesis of the disease (30). Therefore, reduction of Th2 cytokines in the airways by ambroxol may be a cause of the reductions in AHR and airway inflammation induced by allergen. When ambroxol treatment was initiated following completion of the OVA challenges in previously sensitized mice, the development of AHR was reduced compared to the control group and levels of IL-10, IFN-γ, and IL-12 in BAL fluid were significantly increased. The levels of protein carbonyls as a marker of oxidative stress were also significantly reduced. However, the numbers of eosinophils and levels of Th2 cytokines in BAL fluid, and goblet cell metaplasia were not altered under this delayed treatment regimen. Thus, ambroxol appeared to act at two different levels: the prevention of AHR in response to primary allergen challenge and the reduction in AHR following provocative challenge in mice with previously established allergic lung disease. In In vitro cultures, ambroxol treatment increased levels of IL-10, IFN-γ, and IL-12 from lung MNCs and macrophages, whereas levels of the Th2 cytokines, IL-4, IL-5, and IL-13 from lung MNCs were not altered. Based on these data, one possible mechanism underlying the effects of ambroxol on airway function in allergic airway inflammation was the upregulation of IL-10, IFN-γ, and IL-12, cytokines known to suppress AHR and allergic airway inflammation through inhibition of Th2 cytokine production (3132). Thus, ambroxol-mediated effects on Th2 cytokine levels likely were indirect and mediated through upregulation of IL-10, IFN-γ, and IL-12 in the airways. IL-10 and IFN-γ can be produced by a variety of immune cells, but IL-12 is produced primarily by pathogen-activated antigen-presenting cells, particularly macrophages and dendritic cells (33). Together with the inhibitory effects of ambroxol on the secretion of histamine and leukotriene B4 from monocytes (22), the pharmacological target of ambroxol is likely to be a monocyte/macrophage population. When mice were treated with ambroxol prior to OVA challenge, BAL levels of IL-5 and IL-13 were decreased but IL-10, IFN-γ, and IL-12 levels were not altered. This may be explained by the timing of ambroxol treatment; ambroxol treatment prior to allergen challenge may upregulate IL-10, IFN-γ, and IL-12 but this was not detected when BAL samples were collected 3 days after final administration. Thus, one effect of ambroxol on the development of AHR and airway allergic inflammation may be mediated through the upregulation of IL-10, IFN-γ, and IL-12 in monocytes/macrophages.
A second effect of the drug may lie in the anti-oxidant activity. Oxidative stress can be induced in any organ of the body when the balance of anti-oxidant versus reactive oxygen species is altered (34). Oxidative stress causes damage in the airways and exaggerates airway diseases including asthma or in animal models of airway allergic inflammation (3536). In the animal studies, treatment with anti-oxidant reagents or genetic manipulation, the over-expression of Cu/Zn superoxide dismutase or deficiency of nuclear erythroid 2 p45-related factor 2, a transcription factor regulating many antioxidant genes, were shown to be protective in the airways (373839). Ambroxol has been shown to have anti-oxidant properties and reduce oxidant production from inflammatory cells In vitro, such as polymorphonuclear cells, alveolar macrophages, or human bronchial epithelial cells (16404142), and inhibited AHR induced by ozone in dogs (43). In the present study, we demonstrated that ambroxol treatment begun after OVA challenge of sensitized mice decreased the levels of protein carbonyls in BAL fluid, a biomarker of oxidative stress (44). In parallel, airway responsiveness in response to inhaled MCh was decreased while eosinophil numbers were not altered. This may indicate that the anti-oxidant properties of ambroxol target airway function with little to no effect on airway inflammation. Ambroxol treatment prior to OVA challenge did not alter protein carbonyl levels in BAL fluid. The assumption was that the anti-oxidant effects of ambroxol were transient and decreased levels of protein carbonyls in BAL fluid were not observed 2 days after cessation of ambroxol treatment. Similarly, effects on BAL cytokine levels, IL-10, IFN-γ and IL-12, were not seen at this time point.
Recent studies have revealed regulatory effects of oxidative stress on cytokine production. Frossi et al. showed that low doses of H2O2 downregulate IFN-γ In vitro but upregulate IL-4 release from T cells (45). Obata et al. showed that animals treated with H2O2 in vivo developed higher Th2-type responses through production of IL-12 p40 homodimers (46). These findings may explain some of the results seen in this study; the antioxidant properties of ambroxol may be linked to the increased levels of IL-12 in the mice with established airway inflammation but was protective against Th2 differentiation detected as decreased levels of IL-5 and IL-13.
In summary, administration of ambroxol was shown to normalize AHR when given either prior to or after completion of allergen challenge in previously sensitized mice. Ambroxol treatment prior to allergen challenge reduced BAL eosinophils and Th2 cytokines, while the same treatment begun after completion of the allergen challenges also impacted cytokine levels and levels of protein carbonyls in BAL fluid. Taken together, ambroxol exhibits potent therapeutic benefits through its antioxidant and immunomodulatory properties in these animal models and may prove beneficial in asthmatics.
Figures and Tables
Figure 1
Scheme of the experimental protocol. Mice were sensitized and challenged to OVA (OVA/OVA) or sham-sensitized with PBS followed by OVA challenge (PBS/OVA). Ambroxol treatments were administered (A) beginning 2 days before through the OVA challenge days followed by 2 days of rest or (B) initiated after OVA challenges were completed. OVA, ovalbumin.
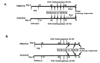
Figure 2
Effect of ambroxol on airway responses when administered prior to initiation of allergen challenge. Mice were sensitized to OVA or PBS followed by aerosolized OVA challenged (OVA/OVA and PBS/OVA, respectively). Ambroxol or vehicle treatment was initiated prior to OVA challenge. (A) Changes in lung resistance (RL). RL values were obtained in response to increasing concentrations of inhaled methacholine (MCh). (B) Cell composition in BAL fluid. (C) Cytokine levels in BAL fluid from mice which received ambroxol or saline treatment prior to OVA challenge. (D) Representative histological sections stained with H/E and PAS: (a) PBS/OVA with vehicle treatment, (b) OVA/OVA with vehicle treatment and (c) OVA/OVA with ambroxol treatment. (E) Quantitative analysis of PAS+ areas on lung histological sections. The results for each group are expressed as mean±SEM (n=8 in each group). *p<0.05; significant differences are shown compared to the groups of OVA/OVA with vehicle treatments. †p<0.05; significant differences are shown compared to the groups of PBS/OVA.
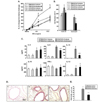
Figure 3
Effect of ambroxol on airway responses when administered after completion of the allergen challenges. Mice were sensitized and challenged to OVA (OVA/OVA) or sham-sensitized followed by OVA challenge (PBS/OVA). Ambroxol or saline were administrated following OVA challenge. (A) Changes in lung resistance (RL) and (B) Cell composition in BAL fluid. (C) Cytokine levels in BAL fluid in mice which received ambroxol or saline following OVA challenge. Mice were sensitized to OVA or PBS (OVA/OVA and PBS/OVA, respectively) followed by OVA challenge. (D) Representative histological sections after H/E and PAS staining: (a) PBS/OVA with vehicle treatment, (b) OVA/OVA with vehicle treatment and (c) OVA/OVA with ambroxol treatment. (E) Quantitative analysis of PAS+ areas. (F) The levels of protein carbonyls in BAL fluid from mice which received ambroxol or vehicle following OVA challenge were measured by ELISA and the levels are expressed as absorbance standardized to 20 µg of protein in BAL fluid. The results for each group are expressed as mean±SEM (n=8). *p<0.05; significant differences are shown compared to the groups of OVA/OVA with vehicle treatments. †p<0.05; significant differences are shown compared to the groups of PBS/OVA.
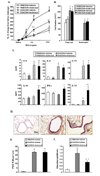
Figure 4
In vitro cytokine production from lung MNCs and alveolar macrophages. Lung MNCs were obtained from mice sensitized and challenged to OVA. Alveolar macrophages were collected from OVA sensitized mice without challenge. The levels of In vitro cytokine production from (A) lung MNCs and (B) alveolar macrophages. Cytokine levels in supernatants from cells cultured with OVA and different concentrations of ambroxol were measured by ELISA as described in Materials and Methods. The results for each group are expressed as mean±SEM (n=8). *p<0.05; significant differences are shown compared to controls; †p<0.05; significant differences are shown compared to OVA treatment alone.
ACKNOWLEDGMENTS
This study was supported by National Institute of Health grant HL-36577 (to E.W.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the NIH. The authors thank Diana Nabighian for assistance in preparing the manuscript.
References
1. Anandan C, Nurmatov U, van Schayck OC, Sheikh A. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy. 2010; 65:152–167.

2. Thomas M. Why aren't we doing better in asthma: time for personalised medicine. NPJ Prim Care Respir Med. 2015; 25:15004.

3. Williams OW, Sharafkhaneh A, Kim V, Dickey BF, Evans CM. Airway mucus: From production to secretion. Am J Respir Cell Mol Biol. 2006; 34:527–536.
4. Lai HY, Rogers DF. Mucus hypersecretion in asthma: intracellular signaling pathways as targets for pharmacotherapy. Curr Opin Allergy Clin Immunol. 2010; 10:67–76.

5. Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, Foster PS, Rothenberg ME. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immunol. 2001; 108:594–601.

7. Ishibashi Y, Kobayashi F, Idesawa A, Taniguchi A, Matsuzawa S. Effects of carbocisteine on altered activities of glycosidase and glycosyltransferase and expression of Muc5ac in SO2-exposed rats. Eur J Pharmacol. 2004; 487:7–15.

8. Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: Central mediator of allergic asthma. Science. 1998; 282:2258–2261.

9. Takeyama K, Fahy JV, Nadel JA. Relationship of epidermal growth factor receptors to goblet cell production in human bronchi. Am J Respir Crit Care Med. 2001; 163:511–516.

10. Malerba M, Ponticiello A, Radaeli A, Bensi G, Grassi V. Effect of twelve-months therapy with oral ambroxol in preventing exacerbations in patients with COPD. Double-blind, randomized, multicenter, placebo-controlled study (the AMETHIST Trial). Pulm Pharmacol Ther. 2004; 17:27–34.

11. Ratjen F, Wonne R, Posselt HG, Stover B, Hofmann D, Bender SW. A double-blind placebo controlled trial with oral ambroxol and N-acetylcysteine for mucolytic treatment in cystic fibrosis. Eur J Pediatr. 1985; 144:374–378.

12. Malerba M, Ragnoli B. Ambroxol in the 21st century: Pharmacological and clinical update. Expert Opin Drug Metab Toxicol. 2008; 4:1119–1129.

13. Paleari D, Rossi GA, Nicolini G, Olivieri D. Ambroxol: a multifaceted molecule with additional therapeutic potentials in respiratory disorders of childhood. Expert Opin Drug Discov. 2011; 6:1203–1214.

14. Melillo G, Cocco G. Ambroxol decreases bronchial hyperreactivity. Eur J Respir Dis. 1986; 69:316–320.
15. Spicak V, Kacirek S, Pohunek P. Treatment of bronchial obstruction in asthmatic children. Bull Eur Physiopathol Respir. 1987; 10:107s–109s.
16. Huang J, Xu J, Tian L, Zhong L. A thioredoxin reductase and/or thioredoxin system-based mechanism for antioxidant effects of ambroxol. Biochimie. 2014; 97:92–103.

17. Seifart C, Clostermann U, Seifart U, Muller B, Vogelmeier C, von Wichert P, Fehrenbach H. Cellspecific modulation of surfactant proteins by ambroxol treatment. Toxicol Appl Pharmacol. 2005; 203:27–35.

18. Seagrave J, Albrecht HH, Hill DB, Rogers DF, Solomon G. Effects of guaifenesin, N-acetylcysteine, and ambroxol on MUC5AC and mucociliary transport in primary differentiated human tracheal-bronchial cells. Respir Res. 2012; 13:98.

19. Laoag-Fernandez JB, Fernandez AM, Maruo T. Antenatal use of ambroxol for the prevention of infant respiratory distress syndrome. J Obstet Gynaecol Res. 2000; 26:307–312.

20. Gonzalez Garay AG, Reveiz L, Velasco Hidalgo L, Solis Galicia C. Ambroxol for women at risk of preterm birth for preventing neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2014; 10:CD009708.

21. Su X, Wang L, Song Y, Bai C. Inhibition of inflammatory responses by ambroxol, a mucolytic agent, in a murine model of acute lung injury induced by lipopolysaccharide. Intensive Care Med. 2004; 30:133–140.

22. Gibbs BF. Differential modulation of IgE-dependent activation of human basophils by ambroxol and related secretolytic analogues. Int J Immunopathol Pharmacol. 2009; 22:919–927.

23. Dong C, Wang G, Li B, Xiao K, Ma Z, Huang H, Wang X, Bai C. Anti-asthmatic agents alleviate pulmonary edema by upregulating AQP1 and AQP5 expression in the lungs of mice with OVA-induced asthma. Respir Physiol Neurobiol. 2012; 181:21–28.

24. Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J Exp Med. 1997; 186:449–454.

25. Takeda K, Miyahara N, Kodama T, Taube C, Balhorn A, Dakhama A, Kitamura K, Hirano A, Tanimoto M, Gelfand EW. S-carboxymethylcysteine normalises airway responsiveness in sensitised and challenged mice. Eur Respir J. 2005; 26:577–585.

26. Ritter C, da Cunha AA, Echer IC, Andrades M, Reinke A, Lucchiari N, Rocha J, Streck EL, Menna-Barreto S, Moreira JC, Dal-Pizzol F. Effects of N-acetylcysteine plus deferoxamine in lipopolysaccharide-induced acute lung injury in the rat. Crit Care Med. 2006; 34:471–477.

27. Houtmeyers E, Gosselink R, Gayan-Ramirez G, Decramer M. Effects of drugs on mucus clearance. Eur Respir J. 1999; 14:452–467.

28. Himmel W, Hummers-Pradier E, Schumann H, Kochen MM. The predictive value of asthma medications to identify individuals with asthma--a study in German general practices. Br J Gen Pract. 2001; 472:879–883.
29. Lemanske RF Jr, Busse WW. Asthma: Factors underlying inception, exacerbation, and disease progression. J Allergy Clin Immunol. 2006; 117:S456–S461.
30. O'Byrne PM, Inman MD, Adelroth E. Reassessing the Th2 cytokine basis of asthma. Trends Pharmacol Sci. 2004; 25:244–248.
31. Schwarze J, Hamelmann E, Cieslewicz G, Tomkinson A, Joetham A, Bradley K, Gelfand EW. Local treatment with IL-12 is an effective inhibitor of airway hyperresponsiveness and lung eosinophilia after airway challenge in sensitized mice. J Allergy Clin Immunol. 1998; 102:86–93.

32. Matsumoto K, Inoue H, Tsuda M, Honda Y, Kibe A, Machida K, Yoshiura Y, Nakanishi Y. Different roles of interleukin-10 in onset and resolution of asthmatic responses in allergen-challenged mice. Respirology. 2005; 10:18–26.

33. Ma X, Yan W, Zheng H, Du Q, Zhang L, Ban Y, Li N, Wei F. Regulation of IL-10 and IL-12 production and function in macrophages and dendritic cells. F1000Res. 2015; 4:pii: F1000 Faculty Rev-1465.

34. Bowler RP, Crapo JD. Oxidative stress in allergic respiratory diseases. J Allergy Clin Immunol. 2002; 110:349–356.

35. Park JW, Taube C, Swasey C, Kodama T, Joetham A, Balhorn A, Takeda K, Miyahara N, Allen CB, Dakhama A, Kim SH, Dinarello CA, Gelfand EW. Complement activation is critical to airway hyperresponsiveness after acute ozone exposure. Am J Respir Crit Care Med. 2004; 169:726–732.

36. Comhair SA, Ricci KS, Arroliga M, Lara AR, Dweik RA, Song W, Hazen SL, Bleecker ER, Busse WW, Chung KF, Gaston B, Hastie A, Hew M, Jarjour N, Moore W, Peters S, Teague WG, Wenzel SE, Erzurum SC. Correlation of systemic superoxide dismutase deficiency to airflow obstruction in asthma. Am J Respir Crit Care Med. 2005; 172:306–313.

37. Blesa S, Cortijo J, Mata M, Serrano A, Closa D, Santangelo F, Estrela JM, Suchankova J, Morcillo EJ. Oral N-acetylcysteine attenuates the rat pulmonary inflammatory response to antigen. Eur Respir J. 2003; 21:394–400.

38. Larsen GL, White CW, Takeda K, Loader JE, Nguyen DD, Joetham A, Groner Y, Gelfand EW. Mice that overexpress Cu/Zn superoxide dismutase are resistant to allergen-induced changes in airway control. Am J Physiol Lung Cell Mol Physiol. 2000; 279:L350–L359.

39. Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005; 202:47–59.

40. Teramoto S, Suzuki M, Ohga E, Ishii T, Matsui H, Matsuse T, Ouchi Y. Effects of ambroxol on spontaneous or stimulated generation of reactive oxygen species by bronchoalveolar lavage cells harvested from patients with or without chronic obstructive pulmonary diseases. Pharmacology. 1999; 3:135–141.

41. Gillissen A, Bartling A, Schoen S, Schultze-Werninghaus G. Antioxidant function of ambroxol in mononuclear and polymorphonuclear cells In vitro. Lung. 1997; 175:235–242.

42. Peroni DG, Moser S, Gallo G, Pigozzi R, Tenero L, Zanoni L, Boner AL, Piacentini GL. Ambroxol inhibits neutrophil respiratory burst activated by alpha chain integrin adhesion. Int J Immunopathol Pharmacol. 2013; 26:883–887.

43. Chitano P, Di Stefano A, Finotto S, Zavattini G, Maestrelli P, Mapp C, Fabbri LM, Allegra L. Ambroxol inhibits airway hyperresponsiveness induced by ozone in dogs. Respiration. 1989; 55:Suppl 1. 74–78.

44. Buss IH, Darlow BA, Winterbourn CC. Elevated protein carbonyls and lipid peroxidation products correlating with myeloperoxidase in tracheal aspirates from premature infants. Pediatr Res. 2000; 47:640–645.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download