Abstract
Helicobacter pylori infection is associated with chronic gastritis, peptic ulcer, and gastric cancer. There is evidence that IL-1β is associated with the development of gastric cancer. Therefore, downregulation of H. pylori-mediated IL-1β production may be a way to prevent gastric cancer. Withaferin A (WA), a withanolide purified from Withania somnifera, is known to exert anti-inflammatory and anti-tumor effects. In the present study, we explored the inhibitory activity of WA on H. pylori-induced production of IL-1β in murine bone marrow-derived dendritic cells (BMDCs) and the underlying cellular mechanism. Co-treatment with WA decreased IL-1β production by H. pylori in BMDCs in a dose-dependent manner. H. pylori-induced gene expression of IL-1β and NLRP3 (NOD-like receptor family, pyrin domain containing 3) were also suppressed by WA treatment. Moreover, IκB-α phosphorylation by H. pylori infection was suppressed by WA in BMDCs. Western blot analysis revealed that H. pylori induced cleavage of caspase-1 and IL-1β, as well as increased procaspase-1 and pro IL-1β protein levels, and that both were suppressed by co-treatment with WA. Finally, we determined whether WA can directly inhibit ac tivation of the NLRP3 inflammasome. NLRP3 activators induced IL-1β secretion in LPS-primed macrophages, which was inhibited by WA in a dose-dependent manner, whereas IL-6 production was not affected by WA. Moreover, cleavage of IL-1β and caspase-1 by NLRP3 activators was also dose-dependently inhibited by WA. These findings suggest that WA can inhibit IL-1β production by H. pylori in dendritic cells and can be used as a new preventive and therapeutic agent for gastric cancer.
Helicobacter pylori (H. pylori) is a gram-negative, spiral shaped bacterium that chronically colonizes the gastric mucosa of more than 50% of the world's human population (1). Gastric cancer is the third leading cause of cancer-related death worldwide (2), and H. pylori infection is considered the main risk factor for development of gastric cancer (3). Progression to gastric cancer has been linked to severe H. pylori-mediated chronic inflammation which is characterized by the recruitment of immune cells, including dendritic cells (DCs), neutrophils, macrophages, and B and T lymphocytes to the site of infection (4).
Interleukin-1β (IL-1β) is a potent pro-inflammatory cytokine that is crucial for host defense against infection and cellular injury (5). IL-1β is produced by various cell types, including monocytes, macrophages, DCs, lymphocytes, neutrophils, fibroblasts, and endothelial cells (6). Increased IL-1β levels have been associated with the development of human diseases, such as atherosclerosis, Alzheimer's disease, type 2 diabetes, and various autoimmune diseases (7). The importance of IL-1β in disease is emphasized by the observation that IL1B gene polymorphisms are associated with a high risk of gastric cancer (8910). Moreover, stomach-specific expression of IL-1β in transgenic mice leads to spontaneous gastric inflammation and cancer (11). Shigematsu et al. showed that recruitment of neutrophils and macrophages by H. pylori infection as well as gastric tumors are significantly suppressed in IL-1 β-deficient mice (12).
The process of IL-1β production is initiated by a wide variety of stimuli, such as pathogen-associated molecular pattern molecules (PAMPs) and damage-associated molecular pattern molecules (DAMPs). Recognition of PAMPs and DAMPs by host pattern recognition receptors such as membrane Toll-like receptors (TLRs) and cytosolic Nod-like receptors (NLRs) activate downstream signaling and subsequently induce the expression of proinflammatory cytokine genes, including pro IL-1β. In most cases, maturation of IL-1β requires enzymatic cleavage by caspase-1, which is activated by various inflammasomes (13). H. pylori is known to induce caspase-1 activation (14) and produce IL-1β in DCs via TLR2/Nod2 and NLRP3 inflammasome-dependent pathways (1516).
Withania somnifera has been used in chronic disease therapies in Ayuvedic medicine of India, and its therapeutic effects are attributed to steroidal lactones known as withanolides. One of these withanolides, Withaferin A (WA) is known to have anti-inflammatory and anti-cancer properties (17181920). WA inhibits iNOS expression and nitric oxide (NO) production in LPS-treated macrophages by downregulating AKT and NF-κB activation (21). In a recent study, we revealed that WA can reduce IL-8 production and NF-κB activation by H. pylori in AGS cells, a human gastric cancer cell line (accepted in Mol Med Rep). In the present study, we investigated the inhibitory effect of WA on H. pylori-induce production of IL-1β in murine bone marrow-derived dendritic cells (BMDCs).
Wild-type C57BL/6 mice were obtained from Koatech (Pyeongtaek, Korea) for the isolation of DCs and macrophages from bone marrow. Animal studies were approved and carried out according to the regulations of the Institutional Animal Care and Use Committee at Konyang University (Daejeon, Korea).
Ultrapure LPS from Escherichia coli O111:B4 was purchased from InvivoGen (San Diego, CA, USA). ATP, Triton X-100, Withaferin A, and nigericin sodium salt were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). Monosodium urate crystals (MSU) were purchased from InvivoGen. Bay 11-7082 (NF-κB inhibitor) was purchased from Calbiochem (La Jolla, CA, USA). H. pylori strain 26695 (ATCC 700392) (American Type Culture Collection, Manassas, VA, USA) was grown on brucella agar (BD Bioscience, Bedford, MA, USA) or brucella broth (BD Bioscience) containing 10% FBS, 10 µg/mL of vancomycin, 5 µg/mL of trimethoprim, and 1 µg/mL of nystatin (Sigma-Aldrich) at 37℃ under microaerobic conditions. For further experiments, the bacteria was prepared in sterile phosphate buffered saline (PBS; pH 7.4) at a concentration of 1×109 bacteria/mL, which was measured by optical density determination at 600 nm and adjusted to a final absorbance of 0.6.
Macrophages (BMDMs) and dendritic cells (BMDCs) derived from murine bone marrow were prepared as previously described (2223). Briefly, BMDCs were cultured with RPMI media containing GM-CSF (20 ng/mL), 1% L-glutamine, 1% penicillin/streptomycin, 10% FBS, and 2-mercaptoethanol (0.1 µg/mL) in a 5% CO2 incubator at 37℃, and fresh media was added on days 3 and 6. After 9 days, non-adherent cells were collected by vigorous aspiration. BMDCs were seeded in 48-well plates at a concentration of 2×105 cells/well for cytokine analysis or in 6-well plates at a concentration of 4×106 cells/well for immunoblotting and real-time PCR analysis. To determine the production of IL-1β, BMDCs were infected with H. pylori strain 26695 at the indicated a multiplicity of infection (MOI) in the absence or presence of WA (100, 250, and 500 nM) for 18 h. BMDMs were cultured in complete Iscove's modified Dulbecco's medium (IMDM, Gibco, Grand Island, NY, USA), with 30% L929 cell culture supernatant, 10% FBS, 1% sodium pyruvate, 1% MEM Non-Essential Amino Acids (MEM NEAA), and 1% penicillin/streptomycin in a 5% CO2 incubator at 37℃. After 3 days, 10 mL of fresh medium was added, and the cells were incubated for an additional 2 days. The cells were seeded in 48-well plates in triplicate at a concentration of 1.6×105 cells/well for cytokine analysis or in 6-well plates at a concentration of 2×106 cells/well for immunoblotting. BMDMs were primed with LPS (1 µg/mL) for 6 h and treated with various doses of WA (40, 100, and 250 nM) for an additional 30 min. Subsequently, the cells were incubated with NLRP3 activators (ATP, nigericin, and MSU) for the indicated times, and culture supernatants were collected to measure the levels of IL-1β and IL-6. Human monocytic leukemia cell line THP-1 (KCTC HC18114, Daejeon, Korea) was cultured in RPMI 1640 medium containing 10% FBS, 1% penicillin/streptomycin in a 5% CO2, 37℃ incubator. To induce differentiation, THP-1 cells were seeded onto 24-well plates at a density of 4×105 cells/well and stimulated with 100 nM phorbol-12-myristate-13-acetate (PMA, Sigma-Aldrich) for 48 h. Plates were washed two times with 500 µL PBS. To determine the production of IL-1β, THP-1 cells were infected with H. pylori strain 26695 at the indicated a multiplicity of infection (MOI 50) in the absence or presence of WA (100, 250, and 500 nM) for 18 h.
The concentrations of IL-6 and IL-1β in culture supernatants were determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA).
BMDCs were infected with H. pylori strain 26695 (MOI 50) with or without WA (500 nM). Culture supernatants were harvested 12 h after infection for further analysis. Cells were lysed at the indicated time points in a buffer containing 10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 5 mM EDTA (pH 8.0), 0.1% Nonidet-P40 supplemented with protease inhibitor (cOmplete, Mini, EDTA-free, Roche, Mannheim, Germany), phosphatase inhibitor (Sigma-Aldrich) and 2 mM dithiothreitol. Proteins from cell-free supernatants were extracted by TCA precipitation (Sigma-Aldrich) and resuspended in 5× sample buffer (1 M Tris, 50% glycerol, 10% SDS, 1% bromophenol blue, and 5% 2-mercaptoethanol). Equal amounts of lysates were loaded onto 12% or 15% polyacrylamide gels, separated by SDS-PAGE, and transferred onto nitrocellulose membranes. Membranes were probed with primary antibodies against regular and phosphorylated forms of IκB-α (Cell signaling Technology, Beverly, MA, USA), IL-1β (R&D Systems), and caspase-1 (AdipoGen, San Diego, CA, USA). A primary antibody against β-actin (Sigma-Aldrich) was used to verify equal loading of protein samples. Following incubation with the relevant secondary antibodies (Santa Cruz Biotechnology, Dallas, TX, USA), proteins were detected with SuperSignal™ West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL, USA). Bands were visualized after exposing the blots to a CP-BU new film (Agfa HealthCare, Mortsel, Belgium). BMDMs were primed with LPS (1 µg/mL) for 6 h and then treated with WA (250 nM) for an additional 30 min. After further incubation with NLRP3 activators [ATP (2 mM), nigericin (10 µM), or MSU (200 µg/mL)], whole cells were lysed with extraction buffer (10% Triton X-100 in PBS supplemented with protease inhibitor and phosphatase inhibitor). Lysates were separated by SDS-PAGE and transferred onto nitrocellulose membranes by immunoblotting. Further experiments followed the same procedures as those in the BMDC experiment.
IL-1β and NLRP3 gene expression levels were determined by real-time PCR. BMDCs were infected with H. pylori strain 26695 (MOI 50) in the absence or presence of WA (500 nM) for 8 h. RNA was extracted using the easy-BLUETM Total RNA Extraction Kit (iNtRON Biotechnology, Seongnam, Korea), and cDNA was prepared from 0.1 µg of RNA using ReverTra Ace® qPCR RT Master Mix (TOYOBO Bio-Technology, Osaka, Japan) according to the manufacturer's instructions. Real-time PCR was performed using the SYBR Green PCR Kit (Qiagen GmbH, Hilden, Germany). GAPDH was used for normalization of expression levels. The following primer sequences were used:
IL-1β (IL-1β forward 5'-GATCCACACTCTCCAGCTGCA-3', IL-1β reverse 5'-CAACCAACAAGTGATATTCTCCATG-3'); NLRP3 (NLRP3 forward 5'-ATGGTATGCCAGGAGGACAG-3', NLRP3 reverse 5'-ATGCTCCTTGACCAGTTGGA-3'); and GAPDH (GAPDH forward 5'-CGACTTCAACAGCAACTCCCACTCTTCC-3', GAPDH reverse 5'-TGGGTGGTCCAGGGTTTCTTACTCCTT-3').
Real-time PCR amplification was performed using a two-step protocol of 95℃ for 10 seconds followed by 58℃ for 45 seconds for 40 cycles in a Rotor-Gene Q real-time PCR system (Qiagen).
The differences among mean values for different groups were tested; values were expressed as the mean±SD. All statistical calculations were performed using GraphPad Prism version 5.01 (GraphPad Software, San Diego, California, USA). We used one-way ANOVA followed by the Bonferroni post-hoc test for multigroup comparisons; a p-value less than 0.05 was considered statistically significant.
Many studies have demonstrated physiological functions of WA, including anti-inflammatory, pro-apoptotic, and anti-proliferative effects (24). Among those, the inhibitory activity of WA on transcriptional factor NF-κB has been well described in various cell lines in response to stimuli such as cytokines and bacterial molecules (e.g., LPS and MDP) (24). Because NF-κB regulates the expression of various proinflammatory cytokine genes in immune cells (25) and H. pylori can lead to IL-1β production in murine BMDCs (1516), we first investigated whether WA inhibits H. pylori-induced production of IL-1β in BMDCs. An MTT assay revealed that WA did not exhibit cytotoxicity in BMDCs at concentrations below 1000 nM (data not shown). H. pylori infection (MOI 5, 10, 25, and 50) induced substantial production of IL-1β in BMDCs, which was significantly inhibited by co-treatment with WA (500 nM) (Fig. 1A). To determine dose-dependency, BMDCs were infected with H. pylori (MOI 50) in the absence or presence of different doses of WA. Results showed that WA treatment reduced IL-1β production by H. pylori in a dose-dependent manner (Fig. 1B). In addition, real-time PCR analysis revealed that WA can inhibit NLRP3 gene expression induced by H. pylori as well as pro IL-1β (Fig. 1C and D), suggesting that WA may have an inhibitory effect on the priming signal of the NLRP3 inflammasome.
The first signal of the inflammasome, often referred to as the priming signal, leads to NF-κB activation and thereby transcription of pro IL-1β for the secretion of mature IL-1β (26). In epithelial cells, H. pylori can induce NF-κB activation via host Nod1 and bacterial type IV secretion system (T4SS)-dependent pathways (2728). Therefore, we sought to determine the kinetics of NF-κB activation by H. pylori in BMDCs and the effect of WA on NF-κB activation. Western blot analysis showed that H. pylori strongly induced IκB-α phosphorylation by 15 min after infection, and that this was sustained at 30 and 60 min (Fig. 1E). H. pylori-induced IκB-α phosphorylation was weakly detected in BMDCs treated with WA at 15 and 30 min after infection and was mostly abolished by 60 min (Fig. 1E). As a positive control, Bay 11-7082 (a selective NF-κB inhibitor) absolutely suppressed H. pylori-induced IκB-α phosphorylation (Fig. 1E). Moreover, Bay 11-7082 reduced IL-1β production by H. pylori in BMDCs in a dose-dependent manner (Fig. 1F). We additionally investigated inhibitory effect of WA on IL-1β production induced by H. pylori in THP-1 cells, a human myeloid leukemia cell line. Consistently, WA inhibited IL-1β production in THP-1 cells dose-dependently (Fig. 1G). These findings suggest that WA may inhibit IL-1β production by immune cells in response to H. pylori infection in an NF-κB-dependent manner.
Caspase-1 is a proteolytic enzyme that cleaves other proteins, such as the precursor forms of IL-1β and IL-18 into active mature forms. Caspase-1 is also activated by proteolytic cleavage, which is mostly driven by inflammasome activation. Recent studies have shown that the NLRP3 inflammasome is essential for H. pylori-mediated caspase-1 activation and IL-1β production in innate immune cells (1516). Accordingly, we explored the effect of WA on caspase-1 activation and IL-1β maturation in BMDCs in response to H. pylori by western blot analysis. As positive controls, BMDCs were primed with LPS for 6 h and subsequently treated with ATP for 40 min. The cells were also infected with H. pylori in the absence or presence of WA (500 nM) for 12 h. LPS priming increased the formation of mature IL-1β, as well as the levels of procaspase-1 and pro IL-1β (Fig. 2), which is consistent with a recent study showing that TLR stimulation leads to NLRP3-mediated IL-1β production independently of the P2X7 receptor in DCs (29). Similarly, H. pylori induced caspase-1 activation and mature IL-1β formation, as well as an increase in procaspase-1 and pro IL-1β protein levels, which was suppressed by co-treatment with WA (Fig. 2). It was remarkable that the production of procaspase-1 and pro IL-1β by H. pylori was partially inhibited by WA, whereas the cleaved forms of caspase-1 and IL-1β were mostly abolished (Fig. 2), suggesting that WA likely affects H. pylori-mediated activation of the NLPR3 inflammasome. Therefore, we next explored whether WA has general inhibitory effects on NLRP3 inflammasome activation. For this experiment, murine BMDMs were primed with LPS and subsequently treated with ATP, nigericin, and MSU to activate the NLRP3 inflammasome in the absence or presence of different doses of WA. Bay 11-7082 was used as a control for NLRP3 inflammasome inhibition (30). Treatment with ATP, nigericin, and MSU led to IL-1β secretion in LPS-primed BMDMs (Fig. 3A-C). IL-1β secretion by NLRP3 activators was decreased by WA in a dose-dependent manner, whereas WA did not affect IL-6 production (Fig. 3A-F). Western blot analysis also showed that ATP, nigericin, and MSU led to cleavage of procaspase-1 and pro IL-1β, which was dose-dependently suppressed by WA as well as Bay 11-7082 (Fig. 4A-C). These results indicate that WA can act as a direct inhibitor of the NLRP3 inflammasome.
An association between polymorphisms of host genetic factors, such as IL-1β, IL-8, and TNF-α, and the risk of developing gastric cancers has been reported (313233). In a human study, the level of IL-1β, but not IL-6, was significantly higher in carcinoma tissues than in normal corresponding gastric mucosa (34). Several studies have also provided evidence that IL-1β contributes to the development of gastric cancer in mouse models. Shigematsu et al. revealed that long-term infection with H. pylori increased IL-1β gene expression in the stomachs of mice (12). IL-1 β-producing cells infiltrates were found in the lamina propria of gastric mucosa of mice infected with H. pylori, whereas no epithelial cells expressed IL-1β (12). Gastric tumors induced by MNU and H. pylori were also suppressed in IL-1β-deficient mice and fewer Ki67-positive cells were found in these mice (12). Moreover, stomach-specific overexpression of IL-1β has been shown to result in the development of gastric inflammation, dysplasia, and carcinoma in transgenic mice and to promote H. felis-induced gastric pathology, which is correlated to the recruitment of myeloid-derived suppressor cells (11). On the other hand, IL-1β seems to be critical for clearance of H. pylori from stomachs. In H. pylori-infected mice, bacterial loads were significantly higher in the stomachs of IL-1β- and IL-1R-deficient mice, as compared with those of WT mice (16). Nevertheless, whether the process of bacterial clearance is beneficial to the host in the case of H. pylori infection is questionable, because H. pylori-induced gastric inflammation was less severe in MyD88- or NLRP3-deficient mice than in WT mice, although a deficiency in MyD88 or NLRP3 led to impaired bacterial clearance from stomachs (1535). Taken together, these results suggest that drugs targeting IL-1β-related signaling may offer new preventive and therapeutic strategies for gastric cancers.
DCs appear to extend into the lumen of gastric glands to take up H. pylori (36) and produce cytokines in response to the bacterium through TLR-mediated signaling (3537). In the present study, we revealed that, in the context of H. pylori infection, WA decreases IL-1β production in DCs by inhibiting NF-κB activation. Furthermore, WA inhibited the NLRP3 inflammasome induced by ATP, nigericin, and MSU, which are well known NLRP3 activators. These findings suggest that WA can inhibit IL-1β production and secretion via dual cellular mechanisms at the steps of both priming (1st signal) and NLRP3 inflammasome assembly (2nd signal). Although it is necessary to clarify the in vivo effect of WA through animal experiments, we suggest that WA may be a new preventive and therapeutic agent for H. pylori-mediated gastric malignancies. In addition, in the present study, we provided evidence of the inhibitory effect of WA only on the NLRP3 inflammasome. Therefore, whether WA exerts an inhibitory effect on other types of inflammasomes, such as NLRC4 and AIM2, should be explored.
Figures and Tables
Figure 1
WA down-regulates H. pylori-mediated IL-1β production and related signaling in immune cells. (A and B) BMDCs were infected with H. pylori at the indicated MOIs (50 in B) for 18 h in the absence or presence of the indicated doses of WA (500 nM in A). The IL-1β levels in culture supernatants were measured by ELISA. (C and D) BMDCs were co-treated with H. pylori (MOI 50) and 500 nM WA for the indicated times, and IL-1β and NLRP3 mRNA expression levels were evaluated by real-time PCR. (E) BMDCs were infected with H. pylori (MOI 50) in the absence or presence of 500 nM of WA or 20 µM of Bay 11 7082. Cellular proteins were harvested at the indicated time points, and the amounts of regular and phospho-form IκB-α and β-actin were determined by western blotting. (F) BMDCs were infected with H. pylori (MOI 50) and treated with various doses of Bay 11-7082 or left untreated for 18 h, and then examined by ELISA. (G) THP-1 cells were also infected with H. pylori at MOI 50 for 18 h in the absence or presence of the indicated doses of WA. The IL-1β levels in culture supernatants were measured by ELISA. Data are shown as the mean±SD of triplicate samples from one representative experiment of three independent experiments (*p<0.5, **p<0.01, and ***p<0.001).
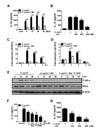
Figure 2
WA reduces the levels of pro and cleaved forms of caspase-1 and IL-1β induced by H. pylori in BMDCs. BMDCs were treated with LPS (100 ng/mL), ATP (5 mM), H. pylori (MOI 50), and WA (500 nM), individually or in combination, as indicated. Pro and cleaved forms of caspase-1 and IL-1β were detected by western blot analysis. β-actin was used as a control for the loading volume.

Figure 3
WA inhibits the production of IL-1β, but not IL-6, by ATP, nigericin, and monosodium urate crystals in BMDMs. BMDMs were primed with LPS (1 µg/mL) for 6 h and subsequently treated with indicated doses of WA or Bay 11-7082 (10 µM). The cells were further incubated with ATP (2 mM) and nigericin (10 µM) for 40 min, or with MSU (200 µg/mL) for 4 h. The levels of IL-1β (A-C) and IL-6 (D-F) in culture supernatants were determined by ELISA.

Figure 4
Caspase-1 activation and IL-1β maturation by NLRP3 activators are suppressed by WA in BMDMs. BMDMs were primed with LPS (1 µg/mL) for 6 h and subsequently treated with the indicated doses of WA or Bay 11-7082 (10 µM). The cells were then incubated with ATP (2 mM) and nigericin (10 µM) for 40 min, or with MSU (200 µg/mL) for 4 h. Pro and cleaved forms of caspase-1 and IL-1β (A-C) was detected by western blot analysis. β-actin was used as a control for the loading volume.

ACKNOWLEDGEMENTS
This work was supported by the Korea Foundation for the Advancement of Science and Creativity (Grant No. SBJ000015942).
References
1. Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002; 347:1175–1186.
2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.

3. Fucci L, Eusebi LH, Bazzoli F. Gastric cancer, Helicobacter pylori infection and other risk factors. World J Gastrointest Oncol. 2010; 2:342–347.
4. Crabtree JE. Gastric mucosal inflammatory responses to Helicobacter pylori. Aliment Pharmacol Ther. 1996; 10:Suppl 1. 29–37.

6. Dinarello CA. The interleukin-1 family: 10 years of discovery. FASEB J. 1994; 8:1314–1325.
7. Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunol Rev. 2011; 243:152–162.

8. Yang J, Hu Z, Xu Y, Shen J, Niu J, Hu X, Guo J, Wei Q, Wang X, Shen H. Interleukin-1B gene promoter variants are associated with an increased risk of gastric cancer in a Chinese population. Cancer Lett. 2004; 215:191–198.

9. Palli D, Saieva C, Luzzi I, Masala G, Topa S, Sera F, Gemma S, Zanna I, D'Errico M, Zini E, Guidotti S, Valeri A, Fabbrucci P, Moretti R, Testai E, del GG, Ottini L, Matullo G, Dogliotti E, Gomez-Miguel MJ. Interleukin-1 gene polymorphisms and gastric cancer risk in a high-risk Italian population. Am J Gastroenterol. 2005; 100:1941–1948.

10. Kumar S, Kumar A, Dixit VK. Evidences showing association of interleukin-1B polymorphisms with increased risk of gastric cancer in an Indian population. Biochem Biophys Res Commun. 2009; 387:456–460.

11. Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-esterreicher M, Bjorkdahl O, Fox JG, Wang TC. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008; 14:408–419.

12. Shigematsu Y, Niwa T, Rehnberg E, Toyoda T, Yoshida S, Mori A, Wakabayashi M, Iwakura Y, Ichinose M, Kim YJ, Ushijima T. Interleukin-1beta induced by Helicobacter pylori infection enhances mouse gastric carcinogenesis. Cancer Lett. 2013; 340:141–147.

13. Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010; 10:210–215.

14. Hitzler I, Sayi A, Kohler E, Engler DB, Koch KN, Hardt WD, Muller A. Caspase-1 has both proinflammatory and regulatory properties in Helicobacter infections, which are differentially mediated by its substrates IL-1beta and IL-18. J Immunol. 2012; 188:3594–3602.

15. Semper RP, Mejias-Luque R, Gross C, Anderl F, Muller A, Vieth M, Busch DH, Prazeres da CC, Ruland J, Gross O, Gerhard M. Helicobacter pylori-induced IL-1beta secretion in innate immune cells is regulated by the NLRP3 inflammasome and requires the cag pathogenicity island. J Immunol. 2014; 193:3566–3576.

16. Kim DJ, Park JH, Franchi L, Backert S, Nunez G. The Cag pathogenicity island and interaction between TLR2/NOD2 and NLRP3 regulate IL-1beta production in Helicobacter pylori infected dendritic cells. Eur J Immunol. 2013; 43:2650–2658.

17. Maitra R, Porter MA, Huang S, Gilmour BP. Inhibition of NFkappaB by the natural product Withaferin A in cellular models of Cystic Fibrosis inflammation. J Inflamm (Lond). 2009; 6:15.

18. Mohan R, Hammers HJ, Bargagna-Mohan P, Zhan XH, Herbstritt CJ, Ruiz A, Zhang L, Hanson AD, Conner BP, Rougas J, Pribluda VS. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis. 2004; 7:115–122.

19. Vyas AR, Singh SV. Molecular targets and mechanisms of cancer prevention and treatment by withaferin a, a naturally occurring steroidal lactone. AAPS J. 2014; 16:1–10.

20. Hahm ER, Singh SV. Withaferin A-induced apoptosis in human breast cancer cells is associated with suppression of inhibitor of apoptosis family protein expression. Cancer Lett. 2013; 334:101–108.

21. Oh JH, Lee TJ, Park JW, Kwon TK. Withaferin A inhibits iNOS expression and nitric oxide production by Akt inactivation and down-regulating LPS-induced activity of NF-kappaB in RAW 2647 cells. . Eur J Pharmacol. 2008; 599:11–17.

22. Celada A, Gray PW, Rinderknecht E, Schreiber RD. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med. 1984; 160:55–74.

23. Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999; 223:77–92.

24. Vanden BW, Sabbe L, Kaileh M, Haegeman G, Heyninck K. Molecular insight in the multifunctional activities of Withaferin A. Biochem Pharmacol. 2012; 84:1282–1291.

25. Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001; 107:7–11.
26. Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013; 13:397–411.

27. Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, Memet S, Huerre MR, Coyle AJ, DiStefano PS, Sansonetti PJ, Labigne A, Bertin J, Philpott DJ, Ferrero RL. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004; 5:1166–1174.

28. Kang MJ, Song EJ, Kim BY, Kim DJ, Park JH. Helicobacter pylori induces vascular endothelial growth factor production in gastric epithelial cells through hypoxia-inducible factor-1alpha-dependent pathway. Helicobacter. 2014; 19:476–483.

29. He Y, Franchi L, Nunez G. TLR agonists stimulate Nlrp3-dependent IL-1beta production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J Immunol. 2013; 190:334–339.

30. Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu JW, Meng R, Quong AA, Latz E, Scott CP, Alnemri ES. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem. 2010; 285:9792–9802.

31. El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni JF Jr, Rabkin CS. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000; 404:398–402.

32. Taguchi A, Ohmiya N, Shirai K, Mabuchi N, Itoh A, Hirooka Y, Niwa Y, Goto H. Interleukin-8 promoter polymorphism increases the risk of atrophic gastritis and gastric cancer in Japan. Cancer Epidemiol Biomarkers Prev. 2005; 14:2487–2493.

33. Hamajima N, Naito M, Kondo T, Goto Y. Genetic factors involved in the development of Helicobacter pylori-related gastric cancer. Cancer Sci. 2006; 97:1129–1138.

34. Kai H, Kitadai Y, Kodama M, Cho S, Kuroda T, Ito M, Tanaka S, Ohmoto Y, Chayama K. Involvement of proinflammatory cytokines IL-1beta and IL-6 in progression of human gastric carcinoma. Anticancer Res. 2005; 25:709–713.
35. Rad R, Brenner L, Krug A, Voland P, Mages J, Lang R, Schwendy S, Reindl W, Dossumbekova A, Ballhorn W, Wagner H, Schmid RM, Bauer S, Prinz C. Toll-like receptor-dependent activation of antigen-presenting cells affects adaptive immunity to Helicobacter pylori. Gastroenterology. 2007; 133:150–163.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download