Abstract
Phellinus linteus has been used as a traditional herbal medicine in Asian countries and is known to have anti-tumor, immunomodulatory, anti-inflammatory, and anti-allergic activities. However, the protective effects of P. linteus against experimental asthma have not been fully investigated. The objective of this study was to determine whether P. linteus ethanol extract (PLE) suppresses inflammatory response in an OVA-induced asthma model. As expected, the oral administration of PLE significantly inhibited eosinophilic airway inflammation and airway hyperresponsiveness in OVA-challenged BALB/c mice. Supporting these data, the augmentation of Th2 cytokines (IL-4, IL-5, and IL-13), eotaxin, and adhesion molecules in lung tissues and bronchoalveolar lavage fluid after OVA inhalation was markedly attenuated by PLE. Furthermore, PLE reduced OVA-induced activation of NF-κB and p38 MAPK in lung tissues. Therefore, our results suggest the potential of P. linteus as a therapeutic agent for asthma.
Hallmarks of allergic asthma include eosinophilic inflammation, reversible airway obstruction, increased mucus production, and airway hyperresponsiveness (AHR) (1). These effects are attributed to Th2 cells and other inflammatory factors including B cells, mast cells, eosinophils, cytokines, and chemokines. In particular, IL-4, IL-5, and IL-13, which are produced by Th2 cells, are related to airway inflammatory changes and AHR through the activation of eosinophils (2). Because the influx and differentiation of Th2 cells contribute to the pathogenesis of asthma, increasing attention has been paid to compounds that target the activation of Th2 cells for the prevention and treatment of asthma.
The transcription factor NF-κB is considered a master regulator of inflammation and immune processes and has been widely implicated in the induction of asthma and other allergic disorders (3). Its increased activity has been observed in allergen-challenged lungs as well as in the airway epithelial cells and macrophages of asthmatic patients (4). According to Choi et al. (5), pretreatment with NF-κB p65 antisense significantly inhibits typical asthmatic features in a murine model. Given these results, the development of a new strategy to inhibit lung-specific NF-κB activity might constitute an interesting topic in the management of asthma.
MAPKs, which are composed of serine and threonine kinases, have critical roles in the activation of inflammatory cells. They are divided into three major subgroups: extracellular signal-related kinase 1/2, p38, and c-Jun N-terminal kinase 1/2 (6). Among them, p38 MAPK is markedly activated in the lungs of asthmatic mice compared with those of normal mice. Blockade of p38 MAPK has been shown to have an anti-inflammatory effect in allergic asthma, suggesting that the inhibition or regulation of p38 MAPK could be essential for minimizing asthmatic symptoms (7).
Phellinus linteus is a well-known fungus in the family Hymenochaetaceae. It has been used as a traditional herbal medicine in Asian countries and reportedly has various pharmacological properties including hepatoprotective, anti-cancer, anti-oxidative, and anti-inflammatory effects (8,9,10). Regarding its anti-allergic activity, we have already shown that P. linteus attenuates mast cell-mediated anaphylaxis-like reactions (11). Consistent with this finding, Lim et al. (12) have demonstrated that organic solvent extracts of P. linteus suppress IgE production by modulating Th1/Th2 balance in murine splenocytes (12). Recently, P. linteus has been revealed to reduce inflammatory skin lesions as well as inhibit serum IgE production in experimental atopic dermatitis (13). These findings favor the hypothesis that P. linteus inhibits allergic airway inflammation in a bronchial asthma model. Nevertheless, to our knowledge, the anti-asthmatic effects of P. linteus and its mechanism of action have not yet been investigated. Thus, the aim of the present study was to investigate whether P. linteus exerts a suppressive effect on pulmonary inflammation and AHR in OVA-induced asthmatic mice.
All experiments were approved by the institutional animal care and use committee of Chonbuk National University School of Medicine and were in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication 82~23, revised 1996) as well as the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines of the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs). All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Dried fruiting bodies of P. linteus were purchased from the collection bank of Huazhong Agricultural University (Hubei, China). Active P. linteus was isolated using ethanol precipitation methods. Briefly, P. linteus was soaked in 50% ethanol, boiled at 90℃ for 4 h, and filtered through a 270-mesh sieve. The sludge from the primary extraction was then extracted twice using the same procedure and evaporated to dryness with a rotary vacuum evaporator (Yiheng Technology Ltd., Shanghai, China) at 40℃ under reduced pressure. The yield was 15% of the raw P. linteus. Chemical analysis revealed that polysaccharides are the major components of PLE, consisting of approximately 72% of the mixture and having an average molecular weight of 1300. Extract was filtered with a 0.2-µm syringe filter and adjusted with distilled water to give a stock solution in 1 mg/ml for experimental use. The solution was stored at 4℃ until use.
Specific pathogen-free, inbred, 7-week-old female BALB/c mice were purchased from Damool Science (Daejeon, Korea). The mice were maintained in an animal facility under standard laboratory conditions for 1 week before experimentation and provided water and standard chow ad libitum. Mice were immunized intraperitoneally with 10µg of OVA (chicken egg albumin, Sigma, St. Louis, MO, USA) plus 1.0 mg of aluminum hydroxide adjuvant (Imject® Alum; Pierce, Rockford, IL, USA). A booster injection of 10µg of OVA plus 1.0 mg aluminum hydroxide adjuvant was given 10 days later. From days 17 to 19, the immunized mice were challenged by exposure to an aerosol of 1% OVA in PBS for 20 min. The mice were divided into groups of seven animals each. Saline-sensitized and challenged mice were used as controls. PLE (10 mg/kg body weight [BW]) dissolved in saline was administered by oral gavage to each animal at 24-h intervals on days 21~23 beginning 1 h before each OVA provocation. An NF-κB inhibitor (BAY 11-7085, 20 mg/kg BW, Sigma) or a p38 MAPK inhibitor (SB 239063, 0.75 mg/kg BW, Sigma) dissolved in sterile DMSO-PBS was injected intraperitoneally into each animal twice on days 21 and 23. In the present study, a vehicle (0.1% v/v DMSO) had no significant effect on key characteristics of allergic asthma (AHR, inflammation, goblet cell metaplasia, and IgE) and important mediators (Th2 cytokines, eotaxin, and adhesion molecules) in an experimental murine model induced by exposure to OVA (data not shown).
AHR was measured 2 days after the last OVA challenge. Conscious, unrestrained mice were placed in a barometric plethysmographic chamber (Synol High-Tech, Beijing, China), and baseline readings were taken and averaged for 3 min. Aerosolized methacholine (Mch) in increasing concentrations (from 2.5 to 50 mg/ml) was then nebulized through an inlet of the main chamber for 3 min, and readings were taken and averaged for 3 min after each nebulization. The bronchopulmonary resistances are expressed as enhanced pauses (Penh), which were calculated as follows: (expiratory time/relaxation time-1)×(peak expiratory flow/peak inspiratory flow), according to the chamber manufacturer's protocol. The results are expressed as the percentage increase in Penh over the baseline after challenge performed with each concentration of Mch, where the baseline Penh (after saline challenge) is expressed as 100%.
Immediately after the assessment of airway responsiveness, mice were anesthetized and their tracheas were cannulated while the thorax was gently massaged. The lungs were lavaged with 0.7 ml of PBS. BAL fluid samples were collected, and the number of total cells in a 0.05-ml aliquot was counted using a hemocytometer (Baxter Diagnostics, Deerfield, IL, USA). The remaining samples were centrifuged, and the supernatants were stored at -70℃ until use for assays of total and OVA-specific IgE, TNF-α, IL-1β, IL-4, IL-5, IL-13, eotaxin, ICAM-1, and VCAM-1 levels. The cell pellets were resuspended in PBS, and cytospin preparations of the BAL cells were stained with Diff-Quik solution (International Reagents, Kobe, Japan). Cell differentials were then enumerated based on cell morphology and staining profiles. Counting was performed by an observer blind to the experimental treatments.
Total and OVA-specific IgE, TNF-α, IL-1β, IL-4, IL-5, IL-13, eotaxin, ICAM-1, and VCAM-1 levels in BAL fluids were determined using mouse ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. The lower limits of detection for the cytokines were as follows (pg/ml): TNF-α, 5.1; IL-1β, 2.0; IL-4, 3.3; IL-5, 5.0; IL-13, 1.5; eotaxin, 3.0; ICAM-1, 0.017; and VCAM-1, 20.
Lungs were fixed with 10% formalin, and the tissues were embedded in paraffin. Fixed tissues were cut into sections 4-µm thick, placed on glass sides, and deparaffinized. Sections were stained with H&E and periodic acid-Schiff for light microscopic examination.
Freshly isolated lung tissues were homogenized in the presence of protease inhibitors, and protein concentrations were determined using the Bradford reagent (Bio-Rad, Hercules, CA, USA). A 30-µg sample of protein from the lung homogenates was loaded per lane on a 12% SDS-PAGE gel for electrophoresis. The proteins were then transferred to nitrocellulose membranes. Western blot analysis was performed using polyclonal antibodies against poly (ADP-ribose) polymerase, β-actin (Santa Cruz Biochemicals, Santa Cruz, CA, USA), phosphorylated (p)-p38, or p38 (R&D Systems). The binding of antibodies was detected using an ECL detection system (iNtRON Biotechnology, Seoul, Korea) according to the manufacturer's instructions.
Cytosolic or nuclear extractions from harvested lung tissues were performed as described previously (14). For western blot analysis, samples were processed using the procedure described above. NF-κB activation was assayed using the antibody against NF-κB p65 (Cell Signaling Technology, Danvers, MA, USA), IκB-α (R&D Systems), or p-IκB-α (Santa Cruz).
To inhibit endogenous protease activity, we added 1 mM PMSF to lung nuclear extracts. An oligonucleotide containing a κ-chain binding site (κB, 5'-CCGGTTAACAGAGGGGGCTTTCCGAG-3') was used as an EMSA probe. The two complimentary strands were annealed and labeled with [α-32P] deoxycytidine triphosphate. Labeled probe (10,000 cpm), 10 µg of nuclear extract, and binding buffer (10 mM Tris-HCl, pH 7.6, 500 mM KCl, 10 mM EDTA, 50% glycerol, 100 ng poly(deoxyinosinic-deoxycytidylic) acid, and 1 mM dithiothreitol) were then incubated for 30 min at room temperature in a final volume of 20µl. Reaction mixtures were analyzed using electrophoresis on 4% polyacrylamide gels in 0.5×Tris-borate buffer. DNA-protein interactions were specific for NF-κB as demonstrated by competition EMSA using a 50-fold excess of unlabeled oligonucleotide (data not shown).
All immunoreactive and phosphorylation signals were analyzed with densitometric scanning (Gel Doc XR; Bio-Rad, Hercules, CA, USA). Data are expressed as means±SEM. Statistical evaluation of the data was performed with ANOVA followed by Dunnett's post-hoc test using Prism 5 software (GraphPad Software, San Diego, CA, USA). Results with a p value of <0.05 were considered statistically significant.
One functional consequence of the inflammatory process that underlies asthma is AHR. In this study, AHR was determined in Penh and was substantially increased in OVA-challenged mice compared with control mice in response to Mch inhalation. PLE, SB 239063, or BAY 11-7085 dramatically prevented AHR in response to inhaled Mch, as shown in Fig. 1A, suggesting that in vivo immune-mediated pathology was modified. Next, to examine the effect of PLE on chemotaxis-that is, the recruitment of inflammatory cells into the airway-we counted inflammatory cells in BAL fluids. We analyzed the cellular composition of the BAL fluids of mice 48 h after the last OVA challenge. In saline-treated mice, OVA challenge resulted in a marked increase in eosinophils and slight increases in neutrophils and lymphocytes compared with counts in control mice (Fig. 1B). However, pretreatment with PLE, SB 239063, or BAY 11-7085 significantly attenuated OVA-induced recruitment of eosinophils (p<0.05). The observed reduction in chemotaxis in the airway correlated with the histological changes in lung parenchyma. Lungs from OVA-challenged mice showed widespread perivascular and peribronchiolar inflammatory cell infiltrates (Fig. 1C and D). Moreover, the percentage of mucus-producing goblet cells (indicated by periodic acid-Schiff staining) in OVA-challenged mice was substantially greater than that in control mice (see Fig. 1C and E). However, administration of PLE significantly reduced inflammatory cell infiltration and goblet cell hyperplasia. These results indicate that PLE efficiently attenuates allergic airway inflammation and mucus hypersecretion in OVA-induced asthmatic mice.
Total and OVA-specific IgE levels were determined with ELISA in each experimental group. IgE levels in BAL fluids were dramatically elevated in OVA-challenged mice compared with those in control mice. However, the administration of PLE or BAY 11-7085 to OVA-challenged mice significantly reduced total and OVA-specific IgE levels (Fig. 2).
Allergic asthmatic inflammation is caused by the secretion of a series of proinflammatory (TNF-α and IL-1β) and Th2 (IL-4, IL-5, and IL-13) cytokines (15). To assess the effect of PLE on pulmonary inflammation in asthmatic mice, we measured the levels of these cytokines in BAL fluids. ELISA showed that the levels of TNF-α, IL-1β, IL-4, IL-5, and IL-13 in BAL fluids were significantly increased in OVA-challenged mice compared with those in control mice (see Fig. 2). These increases were significantly decreased by the administration of PLE or BAY 11-7085. Moreover, given that chemokines and leukocyte-endothelial adhesion molecules are central in the recruitment and migration of leukocytes to the sites of inflammation (16), the levels of eotaxin, ICAM-1, and VCAM-1 were measured. Changes in the levels of these cytokines were similar to those observed for the aforementioned cytokines, indicating that OVA challenge-induced increases in cytokine levels can be reversed by PLE or BAY 11-7085.
In view of the present data that AHR and eosinophilic inflammation in asthmatic mice are blocked by BAY 11-7085, a specific NF-κB inhibitor, and that NF-κB plays a key role in allergic lung inflammation by inducing the transcription of various proinflammatory mediators (3), we hypothesized that PLE attenuates airway inflammatory reactions by suppressing NF-κB activation. To test this hypothesis, we first studied the nuclear translocation of the NF-κB p65 subunit in lung tissues after OVA challenge and observed a decrease and an increase of NF-κB p65 levels in the cytosol and nuclei, respectively, of OVA-challenged lungs (Fig. 3A). By contrast, cytosolic and nuclear extracts from PLE- or SB 239063-treated mice displayed suppressed nuclear translocation.
Next, the effects of PLE on OVA-induced phosphorylation and degradation of IκB-α were evaluated to clarify the molecular mechanisms through which PLE inhibits NF-κB activity. PLE and SB 239063 significantly reduced the OVA-induced phosphorylation and degradation of IκB-α in the cytosol of OVA-challenged lung tissues (Fig. 3B). Furthermore, EMSA revealed an increase in the binding activity of lung nuclear extracts to the NF-κB consensus sequence in OVA-exposed mice (Fig. 3C) compared with control mice, whereas PLE markedly blocked the DNA binding activity of NF-κB. Taken together, these findings indicate that PLE represses NF-κB transcriptional activity, possibly by stabilizing IκB-α and impairing the nuclear transport of the p65 subunit in the lung tissues of OVA-challenged mice.
To investigate whether the inhibition of asthmatic response by PLE is mediated through p38 MAPK, we examined the phosphorylation of p38 MAPK in OVA-challenged mice pretreated with PLE or SB 239063, a p38 MAPK inhibitor. As shown in Fig. 4, PLE and SB 239063 effectively inhibited OVA-induced phosphorylation of p38 MAPK in allergic mice. On the contrary, the expression of p38 was unaffected by OVA, PLE, or SB 239063.
Allergic airway inflammation, characterized by increased infiltration of leukocytes such as eosinophils and considerable secretion of mucus into the airways, is a major factor in the pathogenesis of asthma. In particular, eosinophils have long been recognized as principal effector cells, and they play pathogenic roles in asthma through release of cytotoxic granule proteins (17). Our present findings show that PLE prevents eosinophilic infiltration into the airway, as evidenced by a significant drop in total cell counts and eosinophil counts in BAL fluid.
Likewise, tissue eosinophilia is also inhibited, as revealed by a marked reduction of inflammatory cell infiltration on histological examination. Eosinophilic transmigration into the airways is a multistep process orchestrated by not only Th2 cytokines such as IL-4, IL-5, and IL-13, but also by proinflammatory cytokines including TNF-α and IL-1β and coordinated by a chemotactic cytokine (eotaxin) in combination with adhesion molecules including ICAM-1 and VCAM-1 (18,19). IL-4 is required for B cell maturation and IgE synthesis, and it participates in the initiation of Th2 inflammatory responses. IL-5 is pivotal for growth, differentiation, recruitment, and survival of eosinophils. IL-13 potently induces mucus hypersecretion, eotaxin expression, airway inflammation, and AHR (20,21). TNF-α and IL-1β exert similar responses, which include upregulation of eosinophil chemoattractants and adhesion molecules, recruitment of eosinophils, elevation of cytokine release, and enhancement of AHR (15). According to the current study, PLE attenuates the increased release of Th2 and proinflammatory cytokines, eotaxin, and adhesion molecules into the airway of OVA-challenged mice. From these findings, we speculate that PLE prevents eosinophilic airway inflammation by diminishing the secretion of the aforementioned cytokines into lungs.
AHR is a hallmark clinical symptom of asthma and is defined as an abnormal increase in airflow limitation in response to a provoking stimulus. Although few studies have examined the precise mechanisms through which airway inflammation enhances AHR, the release of various mediators during allergic inflammation is suggestive of their critical role in AHR development (22). For example, IL-5 plays a crucial role in AHR by mobilizing and activating eosinophils, leading to the release of proinflammatory products such as major basic protein and cysteinyl leukotrienes, which are closely associated with AHR (23). Similarly, IL-4 and IL-13 induce AHR in murine asthma models in which cysteinyl-leukotrienes might be causative agents of AHR (24). Moreover, AHR could be brought about by a direct effect of TNF-α on airway smooth muscle (25). Furthermore, IgE-mediated mast cell activation may contribute to AHR by producing a wide array of inflammatory mediators and cytokines (26), which extends the potential importance of our previous results that water extract from P. linteus inhibits IgE-induced mast cell degranulation in suggested mechanisms of AHR (11). As such, the observed reduction of AHR by PLE may be related to a decrease in Th2 cytokine production, tissue eosinophilia, TNF-α levels, and mast cell degranulation by PLE.
The transcription factor NF-κB regulates a wide variety of target genes that encode multiple inflammatory cytokines such as TNF-α, IL-1β, IL-4, IL-5, IL-13, ICAM-1, and VCAM-1, all of which are closely implicated in the pathogenesis of asthma, as mentioned above (3). Our results indicate that PLE exerts anti-NF-κB actions in the lung tissues of OVA-challenged mice. Moreover, suppression of NF-κB activity by BAY 11-7085, a specific inhibitor of NF-κB, not only reduces the levels of the aforementioned cytokines but also ameliorates eosinophilic airway inflammation and AHR in our model in line with the results of a previous report (27). Taken together, these results suggest that the mechanism underlying the anti-asthmatic effects of PLE may be attributed to the inhibition of NF-κB transcriptional activity and subsequent reduction of proinflammatory chemical mediators.
As described previously, p38 MAPK is considered to play a cardinal role in allergic asthma (7). Supporting this contention, our study demonstrates that the augmentation in asthmatic symptoms as well as the phosphorylation of p38 MAPK after OVA inhalation is significantly reduced after the administration of SB 239063, a selective p38 MAPK inhibitor. Moreover, SB 239063 prominently represses NF-κB activity in OVA-challenged lungs, suggesting that p38 MAPK acts upstream of the NF-κB signaling pathway in allergic airway disease. Likewise, PLE dramatically decreases the activity of p38 MAPK and NF-κB in the lung tissues of OVA-challenged mice. Accordingly, these observations encourage the view that the treatment of allergic mice with PLE suppresses p38 MAPK and subsequently disrupts NF-κB activity, reversing the pathophysiologic features of asthma.
Ethanol extraction is the first step in the separation, characterization, and quantification of phenolic compounds from plant materials. As previously described, P. linteus is extracted with 70~80% ethanol and can subsequently be fractionated into aromatic compounds such as hispolon, hispidin, and inotilone, all of which exhibit anti-inflammatory and anti-oxidative effects (28,29,30). Therefore, PLE is likely to contain these agents, which might be the active components responsible for the anti-asthmatic activity of PLE. Future work should be directed toward the purification and identification of the asthma-relieving constituents of P. linteus.
In conclusion, our data indicate that PLE may ameliorate asthmatic inflammation and AHR by downregulating proinflammatory and Th2 cytokines via inhibition of the p38 MAPK-NF-κB module. These findings suggest that P. linteus is a potential anti-inflammatory agent in asthma treatment.
Figures and Tables
Figure 1
Airway hyperresponsiveness, differential cell counts in bronchoalveolar lavage (BAL) fluids, and histological evaluation of lung inflammation after OVA sensitization and treatment with Phellinus linteus ethanol extract (PLE). (A) All animals were nebulized with various concentrations of methacholine as a bronchoconstrictor. Data are shown as the percentage increase in Penh over the baseline, where the baseline Penh of the saline-treated control group is expressed as 100%. (B) Analysis of the effect of PLE, SB 239063, or BAY 11-7085 on OVA-induced differential cell counts in BAL fluid. EOS, eosinophil; NEU, neutrophil; MAC, macrophage; LYM, lymphocyte. (C) Paraffin-embedded lung sections were stained with H&E and periodic acid-Schiff (PAS). Magnification 200×. Bars indicate 50 µm. Data represent three independent experiments. (D) Inflammation scores. Total lung inflammation was defined as the average of the peribronchial and perivascular inflammation scores. (E) Quantitation of airway mucus expression. Sampling was performed 48 h after the last OVA challenge in mice. Results from three independent experiments with seven mice/group are given as means±SEM. SAL+SAL, saline-challenged mice administered saline; OVA+SAL, OVA-challenged mice administered saline; OVA+PLE, OVA-challenged mice administered PLE; OVA+SB 239063, OVA-challenged mice administered SB 239063; OVA+BAY, OVA-challenged mice administered BAY 11-7085. #p<0.05 vs. SAL+SAL; *p<0.05 vs. OVA+SAL.
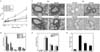
Figure 2
Assessment of IgE, pro-inflammatory and Th2 cytokines, and adhesion molecules in the BAL fluids of OVA-sensitized mice treated with PLE. The levels of IgE, pro-inflammatory (TNF-α and IL-1β) and Th2 (IL-4, IL-5, and IL-13) cytokines, and adhesion molecules (eotaxin, ICAM-1, and VCAM-1) were quantified with ELISA. Sampling was performed 48 h after the last OVA challenge. Results from three independent experiments with seven mice/group are given as means±SEM. SAL+SAL, saline-challenged mice administered saline; OVA +SAL, OVA-challenged mice administered saline, OVA+PLE, OVA-challenged mice administered PLE; OVA+BAY, OVA-challenged mice administered BAY 11-7085. #p<0.05 vs. SAL+SAL; *p<0.05 vs. OVA+SAL.

Figure 3
Effect of PLE on OVA-induced NF-κB activation. The translocation of p65 to the nucleus (A) as well as IκB-α phosphorylation and degradation in cytoplasm (B) and NF-κB DNA binding activity (C) were assessed with western blot and EMSA, respectively. Density ratio vs. β-actin was measured using a densitometer. Results from three independent experiments with seven mice/group are given as means±SEM. SAL+SAL, saline-challenged mice administered saline; OVA +SAL, OVA-challenged mice administered saline; OVA+PLE, OVA-challenged mice administered PLE; OVA+SB 239063, OVA-challenged mice administered SB 239063. #p<0.05 vs. SAL+SAL; *p<0.05 vs. OVA+SAL.
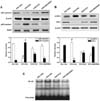
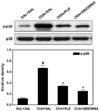
Figure 4
Effect of PLE on OVA-induced p38 mitogen-activate protein kinase (MAPK) activation. Protein expressions of phosphorylated (p)-p38 and p38 in lung tissues were evaluated 48 h after the last OVA challenge. Density ratio vs. β-actin was measured using a densitometer. Results from three independent experiments with seven mice/group are given as means±SEM. SAL+SAL, saline-challenged mice administered saline; OVA+SAL, OVA-challenged mice administered saline; OVA+PLE, OVA-challenged mice administered PLE; OVA+SB 239063, OVA-challenged mice administered SB 239063. #p<0.05 vs. SAL+SAL; *p<0.05 vs. OVA+SAL.
ACKNOWLEDGEMENTS
This paper was supported by the National Natural Science Foundation of China (81260665).
References
1. Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008; 454:445–454.

2. Medoff BD, Thomas SY, Luster AD. T cell trafficking in allergic asthma: the ins and outs. Annu Rev Immunol. 2008; 26:205–232.

3. Imanifooladi AA, Yazdani S, Nourani MR. The role of nuclear factor-kappaB in inflammatory lung disease. Inflamm Allergy Drug Targets. 2010; 9:197–205.
4. Gras D, Chanez P, Vachier I, Petit A, Bourdin A. Bronchial epithelium as a target for innovative treatments in asthma. Pharmacol Ther. 2013; 140:290–305.

5. Choi IW, Kim DK, Ko HM, Lee HK. Administration of antisense phosphorothioate oligonucleotide to the p65 subunit of NF-kappaB inhibits established asthmatic reaction in mice. Int Immunopharmacol. 2004; 4:1817–1828.

6. Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013; 13:679–692.

7. Duan W, Chan JH, McKay K, Crosby JR, Choo HH, Leung BP, Karras JG, Wong WS. Inhaled p38alpha mitogen-activated protein kinase antisense oligonucleotide attenuates asthma in mice. Am J Respir Crit Care Med. 2005; 171:571–578.

8. Uyanoglu M, Canbek M, van Griensven LJ, Yamac M, Senturk H, Kartkaya K, Oglakci A, Turgak O, Kanbak G. Effects of polysaccharide from fruiting bodies of Agaricus bisporus, Agaricus brasiliensis, and Phellinus linteus on alcoholic liver injury. Int J Food Sci Nutr. 2014; in press: http://informahealthcare.com/doi/abs/10.3109/09637486.2013.869796.
9. Han SB, Lee CW, Jeon YJ, Hong ND, Yoo ID, Yang KH, Kim HM. The inhibitory effect of polysaccharides isolated from Phellinus linteus on tumor growth and metastasis. Immunopharmacology. 1999; 41:157–164.

10. Song M, Park DK, Park HJ. Anti-inflammatory effect of Phellinus linteus grown on germinated brown rice on dextran sodium sulfate-induced acute colitis in mice and LPS-activated macrophages. J Ethnopharmacol. 2014; in press: http://dx.doi.org/10.1016/j.jep.2013.12.059.

11. Choi YH, Yan GH, Chai OH, Lim JM, Sung SY, Zhang X, Kim JH, Choi SH, Lee MS, Han EH, Kim HT, Song CH. Inhibition of anaphylaxis-like reaction and mast cell activation by water extract from the fruiting body of Phellinus linteus. Biol Pharm Bull. 2006; 29:1360–1365.

12. Lim BO, Yamada K, Cho BG, Jeon T, Hwang SG, Park T, Kang SA, Park DK. Comparative study on the modulation of IgE and cytokine production by Phellinus linteus grown on germinated brown Rice, Phellinus Linteus and germinated brown rice in murine splenocytes. Biosci Biotechnol Biochem. 2004; 68:2391–2394.

13. Hwang JS, Kwon HK, Kim JE, Rho J, Im SH. Immunomodulatory effect of water soluble extract separated from mycelium of Phellinus linteus on experimental atopic dermatitis. BMC Complement Altern Med. 2012; 12:159.

14. Lee KS, Kim SR, Park HS, Park SJ, Min KH, Lee KY, Choe YH, Hong SH, Han HJ, Lee YR, Kim JS, Atlas D, Lee YC. A novel thiol compound, N-acetylcysteine amide, attenuates allergic airway disease by regulating activation of NF-kappaB and hypoxia-inducible factor-1alpha. Exp Mol Med. 2007; 39:756–768.

15. Boyce JA, Bochner B, Finkelman FD, Rothenberg ME. Advances in mechanisms of asthma, allergy, and immunology in 2011. J Allergy Clin Immunol. 2012; 129:335–341.

16. Ulbrich H, Eriksson EE, Lindbom L. Leukocyte and endothelial cell adhesion molecules as targets for therapeutic interventions in inflammatory disease. Trends Pharmacol Sci. 2003; 24:640–647.

17. Jacobsen EA, Ochkur SI, Lee NA, Lee JJ. Eosinophils and asthma. Curr Allergy Asthma Rep. 2007; 7:18–26.

19. Mattes J, Foster PS. Regulation of eosinophil migration and Th2 cell function by IL-5 and eotaxin. Curr Drug Targets Inflamm Allergy. 2003; 2:169–174.

20. Li L, Xia Y, Nguyen A, Lai YH, Feng L, Mosmann TR, Lo D. Effects of Th2 cytokines on chemokine expression in the lung: IL-13 potently induces eotaxin expression by airway epithelial cells. J Immunol. 1999; 162:2477–2487.
22. Cockcroft DW, Davis BE. Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol. 2006; 118:551–559. quiz 560-561.

23. Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000; 105:651–663.

24. Vargaftig BB, Singer M. Leukotrienes mediate murine bronchopulmonary hyperreactivity, inflammation, and part of mucosal metaplasia and tissue injury induced by recombinant murine interleukin-13. Am J Respir Cell Mol Biol. 2003; 28:410–419.

25. Brightling C, Berry M, Amrani Y. Targeting TNF-alpha: a novel therapeutic approach for asthma. J Allergy Clin Immunol. 2008; 121:5–10. quiz 11-12.
26. Mayr SI, Zuberi RI, Zhang M, de Sousa-Hitzler J, Ngo K, Kuwabara Y, Yu L, Fung-Leung WP, Liu FT. IgE-dependent mast cell activation potentiates airway responses in murine asthma models. J Immunol. 2002; 169:2061–2068.

27. Kim SR, Lee KS, Park SJ, Min KH, Lee MH, Lee KA, Bartov O, Atlas D, Lee YC. A novel dithiol amide CB3 attenuates allergic airway disease through negative regulation of p38 mitogen-activated protein kinase. Am J Respir Crit Care Med. 2011; 183:1015–1024.

28. Chen YS, Lee SM, Lin CC, Liu CY. Hispolon decreases melanin production and induces apoptosis in melanoma cells through the downregulation of tyrosinase and microphthalmia-associated transcription factor (MITF) expressions and the activation of caspase-3, -8 and -9. Int J Mol Sci. 2014; 15:1201–1215.

29. Chen W, Feng L, Huang Z, Su H. Hispidin produced from Phellinus linteus protects against peroxynitrite-mediated DNA damage and hydroxyl radical generation. Chem Biol Interact. 2012; 199:137–142.

30. Huang GJ, Huang SS, Deng JS. Anti-inflammatory activities of inotilone from Phellinus linteus through the inhibition of MMP-9, NF-kappaB, and MAPK activation in vitro and in vivo. PLoS One. 2012; 7:e35922.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download