Abstract
Transforming growth factor-β (TGF-β) is a highly pleiotropic cytokine playing pivotal roles in immune regulation. TGF-β facilitates tumor cell survival and metastasis by targeting multiple cellular components. Focusing on its immunosuppressive functions, TGF-β antagonists have been employed for cancer treatment to enhance tumor immunity. TGF-β antagonists exert anti-tumor effects through #1 activating effector cells such as NK cells and cytotoxic CD8+ T cells (CTLs), #2 inhibiting regulatory/suppressor cell populations, #3 making tumor cells visible to immune cells, #4 inhibiting the production of tumor growth factors. This review focuses on the effect of TGF-β on T cells, which are differentiated into effector T cells or newly identified tumor-supporting T cells.
Transforming growth factor-β (TGF-β) superfamily consists of a large family of structurally related cytokines, which are classified into two main groups: TGF-β/Activin and bone morphogenetic proteins (BMPs) and growth/differentiation factors (GDFs) (1).
Among them, TGF-β has been well characterized as a representative potent immunosuppressive cytokine. TGF-β has been reported to inhibit immune system by modulating both effectors and suppressors. TGF-β directly inhibits innate immune cells such as macrophages and NK cells and adoptive immune cells such as CD4+ helper T cells and CD8+ cytotoxic T cells, while expanding immunosuppressive regulatory T cells (Tregs) and inducing the recruitment of immunosuppressive myeloid-derived suppressor cells (2).
The immune surveillance system is one of the most important defense mechanisms against cancer progression. Attempts to enhance tumor immunity by various strategies have been tried as supportive anti-cancer therapies. The most potent immunosuppressive cytokine, TGF-β is massively produced and activated in tumor microenvironment, which allows invasive metastatic cancers to escape from immune surveillance (3). Thus, antagonizing TGF-β's functions has been implicated as the most potent anti-tumor immune therapy and some TGF-β antagonists have been on preclinical and clinical trials (4-6).
Tumor recurrence by remote metastasis affects the prognosis of cancer patients after radical surgery. Cancer staging is evaluated by TNM classification, which describes the size and the extent of the Tumor, degree of the spread to regional lymph Nodes and the presence of Metastasis. TNM staging is widely employed as a global standard to plan the treatments and to indicate the prognosis (7). Although there are no detailed parameters in metastasis defined in TNM staging, the size and the number of metastasis significantly affect the prognosis of the patients. In pulmonary metastasis from breast cancer, it has been reported that the size and the number of metastasis significantly correlate with prognosis and re-recurrence and the pulmonary metastasectomy is justified in case of small number of metastasis lesions (8). It has been reported that an increase in tumor size and an increase in number of regional lymph nodes with metastasis were associated with diminution in cellular immunity (9), which might be the consequence of the production of TGF-β by tumors themselves and tumor infiltrating immunosuppressive cells (10,11). Thus, one might expect that the number and the size of tumor and metastasis could be reduced by blocking TGF-β to achieve better prognosis.
Although some tumors express tumor specific antigens, which can be recognized by immune system, tumor specific immune responses often fail to eradicate tumors because tumors evade immune surveillance by variety of strategies. Tumors themselves, tumor stromal cells and the myeloid-derived suppressor cells produce large amount of TGF-β, which is activated in the tumor microenvironment (12-14). By its potent immunosuppressive effects, TGF-β strongly inhibits anti-tumor immunity.
Partly due to TGF-β, loss or down-regulation of MHC class I molecules is frequently observed in malignant cells, which makes them invisible to immune system (15). Poorly immunogenic tumors with low expression of MHC class I can escape from attack by cytotoxic CD8+ T cells but they become more sensitive to attack by NK cells (16). TGF-β also affects the tumor cell-NK cell interaction by modulating the expression of MHC class I homologues on the tumor cells and their receptors on NK cells. Expression of MHC class I homologues such as MHC class-I-chain-related protein A (MICA) and Rae-1γ human and murine NKG2D ligand, respectively, as well as NKG2D are reported to be down-regulated by TGF-β (17-19) Nam JS et al. demonstrated that anti-TGF-β antibody treatment up-regulated NKG2D expression on CD8+ T cells, not NK cells, which were already highly activated even without anti-TGF-β antibody treatment (19). Anti-TGF-β antibody treatment exerts anti-tumor efficacy by enhancing tumor-immune cell interaction (Fig. 1).
TGF-β directly suppresses functions, migration and expansion of cytotoxic CD8+ T cells, NK cells, NK T cells and γδ T cells, while inducing Foxp3+ Tregs (2). In poorly immunogenic syngeneic 4T1 mammary tumor metastasis model, TGF-β does not significantly affect the infiltration of NK cells and Foxp3+ Tregs, whereas depletion of CD8+ T cells abolished the effect of anti-TGF-β antibody treatment. Treatment with anti-TGF-β antibody increases the infiltration of activated CD8+ T cells expressing CD122, cytotoxic effector molecules such as perforin and granzyme B and Fas ligand in metastatic lesions (19,20).
It is remarkable that CD8+ T cells mainly impact the size of tumor metastasis, whereas NK cells dramatically impact metastasis number in poorly immunogenic 4T1 mammary tumor metastasis model. CD8+ T cells do not significantly affect the number of metastasis. Treatment with anti-TGF-β antibody restores the cytolytic CD8+ T cell activity similar to NK cell activity to reduce tumor metastasis number to some extent, although very highly activated NK cells are not significantly affected by anti-TGF-β antibody (19). When TGF-β antagonists are administered systemically, untoward effects by autoimmune reactions might be possible (2). However, anti-TGF-β antibody increases cytotoxic T cell activity only locally at the tumor sites without activating systemic immune system, which indicates that the clinical application of TGF-β antagonists to cancer patients is tolerable (4-6,19,20).
The balance between the effector cell populations and the suppressor cell populations finely tunes immune system. In malignant tumor environment, the suppressor populations such as Foxp3+ Tregs and suppressor myeloid cells dominate over effector cell populations and suppress the effective anti-tumor immunity. Large amount of TGF-β produced and activated in tumor environment promotes the development and migration of these suppressor populations (21,22).
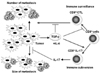
Recently, the novel subset of CD8+ T cells is identified, which promotes growth of primary and metastatic tumor (23). This CD8+ T cell subset is distinct from the reported CD8+ suppressor/regulatory T cells (24). IL-17 is produced by this subset, which favors tumor growth by preventing tumor apoptosis. The same combination of cytokines to induce Th17 CD4+ T cells in vitro, TGF-β and IL-6, subverts CD8+ T cells into this IL-17 producing CD8+ T cells (Fig. 2).
The importance of IL-17 in inflammatory autoimmune disorders was first addressed by Nakae S et al. (25) and the CD4+ T cell subset producing IL-17 was defined as Th17 by Stockinger B et al. (26). Although T cells infiltrated in tumors produce IL-17 (27), it has been controversial whether IL-17 promotes or inhibits tumor growth (28,29). Recently, it is reported that IL-17 produced by CD4+ T cells, not CD8+ T cells, promotes primary tumor growth through IL-6-Stat3 signaling pathway (30).
In poorly immunogenic 4T1 metastatic mammary tumor model, CD8+ T cell depletion decreases the primary tumor volume as well as metastasis size, indicating that some CD8+ T cells are not cytotoxic, but on the contrary, favor the tumor growth (23). IL-17 produced by tumor infiltrating CD8+ T cells was identified as the major soluble factor to prevent tumor cell apoptosis, and the knockdown of IL-17 response of tumor cells reduces tumor growth by enhancing apoptosis in vivo. IL-17 alone or in combination with TGF-β suppresses apoptosis of tumor cells under the condition with nutrient deprivation or the treatment with apoptosis inducer. It might not be universal phenomena for all the malignant tumors, because some tumor cell lines do not respond to IL-17 and in some tumors, IL-17 oppositely induces apoptosis. However, some patients exhibiting the up-regulation of IL-17 expression in tumor (23,27) might benefit from anti-TGF-β treatment through the same mechanism.
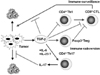
It has been reported that IL-23 is required for autoimmune inflammation mediated by Th17 cells in vivo. It is consistent with the results of lower numbers of IL-17+ T cells in IL-23 deficient mice. These findings suggest that IL-23-dependent signaling in Th-17 cells may depend on the transcription factor Stat3 (31).
Similarly, it is recently reported that Stat3 promotes IL-23-mediated procarcinogenic immune response while inhibiting IL-12-dependent antitumor immunity (32). However, it is not yet determined whether IL-23 is required for the development of IL-17-producing CD4+ T cells and IL-17-producing CD8+ T cells infiltrated in tumor in vivo. They also report that Stat3 is phosphorylated through IL-23 receptor to up-regulate the expression of Foxp3 and immunosuppressive cytokine, IL-10 in CD4+ T cells (Fig. 3). It is also to be determined whether TGF-β is involved in this effect. The IL-17/IL-23 axis might be the new mechanism of tumor immune subversion (33).
Researchers used to focus on tumor immune surveillance by effector cell populations to enhance tumor immunity. Recent accumulation of data shows the importance of regulating tumor immune subversion by suppressor cell populations to reverse immune suppression against tumor. Antagonizing TGF-β successfully enhances tumor immune surveillance and reverses immune subversion, which results in the decrease in the number of metastasis and the size of tumor and metastasis. So far, TGF-β antagonists are applied to the treatment of terminal stage patients of certain highly malignant cancers only in clinical trials. By further confirmation of their efficacy and little toxicity, their application might be expanded to the patients with general cancers before the radical surgery to decrease the size of primary tumors and to prevent metastasis or to the patients before the metastasectomy to reduce the size and the number of metastasis.
Figures and Tables
Figure 1
TGF-β down-regulates the expression of NKG2D on CD8+ T cells and NKG2D ligand on tumor cells.
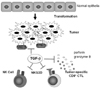
Figure 2
The role of TGF-β in subverting the CD8+ T cell response. TGF-β produced and activated in the tumor environment suppresses the immune surveillance, which mainly affects the number of metastasis by preventing the generation of cytotoxic T cells. On the other hand, TGF-β in combination with other factors, such as IL-6, subverts CD8+ T cells into IL-17 producing cells, which mainly affects the size of tumor and metastasis. IL-17 might promote survival of tumor cells in the condition with low nutrition or chemotherapy.
ACKNOWLEDGEMENTS
We thank Dr. Jeong-Seok Nam, Jin-Ah Park, Ji-Hyun Park and the members of the Laboratory of Immunology for helpful discussions.
References
1. Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol. 2001. 187:265–276.
2. Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006. 24:99–146.
3. Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A. 2003. 100:8621–8623.
4. Yang YA, Dukhanina O, Tang B, Mamura M, Letterio JJ, MacGregor J, Patel SC, Khozin S, Liu ZY, Green J, Anver MR, Merlino G, Wakefield LM. Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J Clin Invest. 2002. 109:1607–1615.

5. Ge R, Rajeev V, Ray P, Lattime E, Rittling S, Medicherla S, Protter A, Murphy A, Chakravarty J, Dugar S, Schreiner G, Barnard N, Reiss M. Inhibition of growth and metastasis of mouse mammary carcinoma by selective inhibitor of transforming growth factor-beta type I receptor kinase in vivo. Clin Cancer Res. 2006. 12:4315–4330.

6. Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007. 13:5262–5270.

7. Denoix PF. Enquete permanent dans les centres anticancereaux. Bull Inst Nat Hyg. 1946. 1:70–75.
8. Chen F, Fujinaga T, Sato K, Sonobe M, Shoji T, Sakai H, Miyahara R, Bando T, Okubo K, Hirata T, Toi M, Date H. Clinical features of surgical resection for pulmonary metastasis from breast cancer. Eur J Surg Oncol. 2009. 35:393–397.

9. Humphrey LJ, Singla O, Volenec FJ. Immunologic responsiveness of the breast cancer patient. Cancer. 1980. 46:893–898.

10. Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005. 202:919–929.

11. Alleva DG, Burger CJ, Elgert KD. Tumor-induced regulation of suppressor macrophage nitric oxide and TNF-alpha production. Role of tumor-derived IL-10, TGF-beta, and prostaglandin E2. J Immunol. 1994. 153:1674–1686.
12. Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006. 6:506–520.
13. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008. 454:436–444.

14. Yang L, Moses HL. Transforming growth factor beta: tumor suppressor or promoter? Are host immune cells the answer? Cancer Res. 2008. 68:9107–9111.

15. Geiser AG, Letterio JJ, Kulkarni AB, Karlsson S, Roberts AB, Sporn MB. Transforming growth factor beta 1 (TGF-beta 1) controls expression of major histocompatibility genes in the postnatal mouse: aberrant histocompatibility antigen expression in the pathogenesis of the TGF-beta 1 null mouse phenotype. Proc Natl Acad Sci U S A. 1993. 90:9944–9948.

16. Ljunggren HG, Kärre K. In search of the 'missing self: MHC molecules and NK cell recognition. Immunol Today. 1990. 11:237–244.

17. Zwirner NW, Fuertes MB, Girart MV, Domaica CI, Rossi LE. Cytokine-driven regulation of NK cell functions in tumor immunity: role of the MICA-NKG2D system. Cytokine Growth Factor Rev. 2007. 18:159–170.

18. Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004. 172:7335–7340.

19. Nam JS, Terabe M, Mamura M, Kang MJ, Chae H, Stuelten C, Kohn E, Tang B, Sabzevari H, Anver MR, Lawrence S, Danielpour D, Lonning S, Berzofsky JA, Wakefield LM. An anti-transforming growth factor beta antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Res. 2008. 68:3835–3843.

20. Thomas DA, Massagué J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005. 8:369–380.

21. Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Semin Cancer Biol. 2006. 16:115–123.

22. Kitamura T, Kometani K, Hashida H, Matsunaga A, Miyoshi H, Hosogi H, Aoki M, Oshima M, Hattori M, Takabayashi A, Minato N, Taketo MM. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet. 2007. 39:467–475.

23. Nam JS, Terabe M, Kang MJ, Chae H, Voong N, Yang YA, Laurence A, Michalowska A, Mamura M, Lonning S, Berzofsky JA, Wakefield LM. Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res. 2008. 68:3915–3923.

25. Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002. 17:375–387.

26. Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGF beta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006. 24:179–189.

27. Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17- producing T cells in ovarian cancer. Proc Natl Acad Sci U S A. 2008. 105:15505–15510.

28. Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003. 101:2620–2627.

29. Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009. 114:357–359.

30. Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009. 206:1457–1464.

31. McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009. 10:314–324.

32. Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, Yu H. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009. 15:114–123.

33. Martin-Orozco N, Dong C. The IL-17/IL-23 axis of inflammation in cancer: friend or foe? Curr Opin Investig Drugs. 2009. 10:543–549.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download