Abstract
Background
Dexmedetomidine is a highly selective central α2-agonist used as a sedative in pediatric intensive care unit (PICU). However, little is known about the relationship between dexmedetomidine dose and its plasma concentration during long-term infusion. We have previously demonstrated that the sedative plasma dexmedetomidine concentration is moderately correlated with the administered dose in adults (r = 0.653, P = 0.001). We hypothesized that there would be a similar relationship between the sedative dexmedetomidine concentration and administered dose in infants.
Methods
All patients admitted to the PICU at Nagoya City University Hospital, Japan, between November 2012 and March 2013 were eligible for inclusion in the study. Plasma dexmedetomidine concentration was measured by ultra-performance liquid chromatography coupled with tandem mass spectrometry.
Results
We measured the plasma dexmedetomidine concentration in 203 samples from 45 patients. Of these, 96 samples collected from 27 patients < 2 years old were included in this study. All patients received dexmedetomidine at 0.12–1.40 µg/kg/h. The median administration duration was 87.6 hours (range: 6–540 hours). Plasma dexmedetomidine concentration ranged from 0.07 to 3.17 ng/ml. Plasma dexmedetomidine concentration was not correlated with the administered dose (r = 0.273, P = 0.007). The approximate linear equation was y = 0.690x + 0.423.
Dexmedetomidine is a highly selective central α2-agonist with anesthetic and analgesic properties used in critically ill pediatric patients [12]. Dexmedetomidine has been used in children with heart disease [3456], those undergoing mechanical ventilation [7], and as part of an early extubation strategy [8]. A long duration of administration may be required [5]. However, dexmedetomidine causes bradycardia, hypotension, and hypertension [910]; it was reported to be responsible for sinus arrest in a healthy young adult volunteer [11] and bradycardia followed by asystole in a 52-year-old woman undergoing thymectomy [12]. In children, the extent of these hemodynamic variations was reported to be related to the serum dexmedetomidine concentration [13]. A comprehensive understanding of the pharmacological, pharmacokinetic, and pharmacodynamic effects of dexmedetomidine, and the best means of maintaining optimal plasma dexmedetomidine concentration, are critical for its safe and efficacious use in pediatric perioperative and critical care settings [14].
The recommended dose of dexmedetomidine for children in the pediatric intensive care unit (PICU) is intravenous infusion of 0.12–1.4 µg/kg/h. There are no methods for simulating blood dexmedetomidine concentration. We have shown previously that a dexmedetomidine dose of 0.20–0.83 µg/kg/h achieved effective sedation in adults, with a plasma dexmedetomidine concentration of 0.22–2.50 ng/ml; dexmedetomidine concentration was moderately correlated with the administered dose (r = 0.653, P < 0.01) [15]. The relationship between dexmedetomidine dose and plasma concentration during long-term infusion in critically ill children is not completely understood [1617].
Our primary aim was to investigate whether the correlation coefficient between the sedative dose of dexmedetomidine and its plasma concentration in infants would exceed 0.5. We conducted a prospective observational cohort study of infants admitted to the PICU at our institution.
This study was conducted in the PICU of Nagoya City University Hospital, Japan. The name of the registry site was the University Hospital Medical Information Network Clinical Trials Registry (unique trial number: UMIN000009115). Ethical approval for the study was provided by the Ethics Committee of Nagoya City University Hospital, Nagoya, Japan, in July 2012 (chair Professor Y. Fujii, reference number 688). The requirement for written informed consent from participants' parents or caregivers was waived by the Ethics Committee, as we used blood samples < 0.5 ml that would otherwise have been discarded after routine blood gas analysis every morning. Nevertheless, we explained the study to the infants' parents or caregivers and recorded their agreement to participate in the medical records.
We recruited consecutive children < 2 years old admitted to the PICU of our institution between November 2012 and March 2013. We excluded infants in whom the dose of dexmedetomidine was changed within 6 hours. Dexmedetomidine was not administered to patients with a history of intolerance to dexmedetomidine, or with significant metabolic, hematological, or endocrine disease.
Patients received continuous intravenous infusion of dexmedetomidine (Precedex®; Hospira Japan, Osaka, Japan) at 0.12–1.4 µg/kg/h without a loading dose. Dexmedetomidine was administered based on clinical need, and all other standard PICU care needs were met. Standard care included intravenous administration of fentanyl or morphine as an analgesic, potentially supplemented by midazolam as a sedative. The oral sedative-hypnotic agents diazepam, triclofos, clonidine, and phenobarbital were also used in selected patients at the discretion of the supervising physician. Sedation was assessed using the Richmond Agitation-Sedation Scale (RASS) as follows: +4, combative; +3, very agitated; +2, agitated; +1, restless; 0, alert and calm; −1, drowsy; −2, light sedation; −3, moderate sedation; −4, deep sedation; −5, unarousable. Arterial blood gas analysis was routinely performed (once daily between 05:00 and 07:00). Blood that would otherwise have been discarded was collected immediately in EDTA tubes. Samples were stored at 4℃ and centrifuged. Plasma was frozen at −80℃ after separation and stored until analysis.
Measurement of dexmedetomidine concentration was performed by a single blind method. Arterial blood samples were numbered and no clinical information, including on whether dexmedetomidine had been administered, was provided to the investigators performing the assays. We have established a method to measure dexmedetomidine concentration using only 10 µl of plasma [18]. Briefly, dexmedetomidine was quantified using the original stable dexmedetomidine-d3 for electrospray ionization-tandem mass spectrometry (ESI-MS/MS) in the selected reaction monitoring mode. A rapid, ultra-high-performance liquid chromatography technique was adopted using ESI-MS/MS with a runtime of 3 minutes. Samples were prepared using a solid-phase extraction technique with an OASIS-HLB cartridge. Recovery values ranged from 99.8% to 100.3% for the intra-day (relative standard deviation [RSD] 0.9–1.3%) and inter-day (RSD 0.9–1.5%) assays. A stable test was defined as having recovery values in the range 97.8–99.7%, with an RSD 1.0–1.9% for the process/wet extract, bench-top, freeze-thaw, and long-term tests. We judged that an efficacious plasma concentration lay in the range 50 pg/ml to 5 ng/ml. The lower limit of quantification was 5 pg/ml.
Power analysis was calculated for the primary endpoint, the correlation coefficient between dexmedetomidine dose and plasma dexmedetomidine concentration. Our preliminary data (sample number = 43) estimated the correlation coefficient to be 0.42. A total sample size of 48–82 was judged to be necessary to obtain a correlation coefficient of 0.35–0.45 and detect the primary outcome with statistical significance, assuming a two-tailed type I error of 5% and type II error of 10%. The Kolmogorov–Smirnov test was used to assess the distribution of data. Data are presented as means ± SD for normally distributed variables, the median [interquartile range, IQR] for non-normally distributed variables, or S the number (proportion, %), as appropriate. The relationship between dexmedetomidine dose and its plasma concentration was determined by linear regression. The correlation coefficient was calculated with Pearson's r or Spearman's ρ, according to the type of distribution. All P values are two-tailed. In all analyses, P < 0.05 was taken to indicate statistical significance.
We also assessed the relationship between dexmedetomidine dose and its plasma concentration in a variety of subgroups: patients undergoing single- or two-ventricle cardiac surgery; patients stratified according to Risk Adjustment in Congenital Heart Surgery (RACHS-1) score (comparing those with RACHS scores of 1–3 to those with scores of 4–6); and patents grouped according to the curative or palliative surgical strategy and age. Finally, we measured the correlation coefficient in patients from whom more than five samples were obtained.
All statistical analyses were performed using SPSS software (ver. 19.0; SPSS Inc., Chicago, IL, USA).
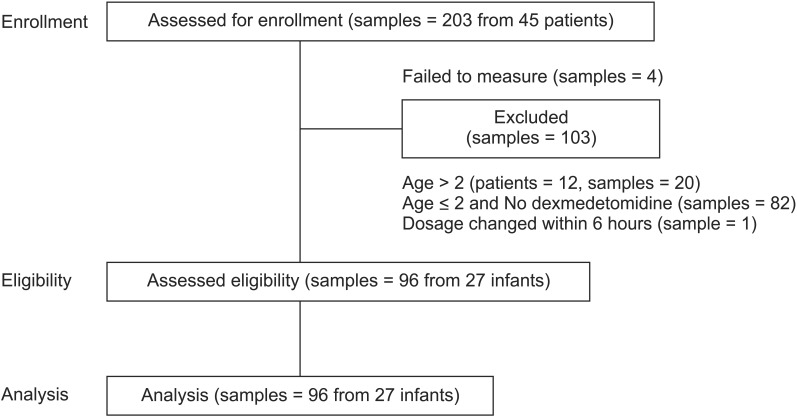
After exclusions, we collected 96 samples from 27 patients; plasma dexmedetomidine concentration was measured after assay failure in four specimens (Fig. 1). The demographic and clinical characteristics of the patients are shown in Table 1. All patients received dexmedetomidine at 0.12–1.40 µg/kg/h (Table 2) and all patients had cardiac disease: 24 had undergone cardiac correction surgery, and the remaining 3 had undergone non-cardiac surgery after previous cardiac repair. Of those that had undergone previous cardiac surgery, one patient underwent Nissen fundoplication having previously undergone the Jatene procedure for complete transposition of the great arteries, one underwent surgery for imperforate anus having undergone the Glenn procedure for double-outlet right ventricle, and the other underwent cleft lip repair after ligation of a patent ductus arteriosus.
The dexmedetomidine dose had not been changed for at least 6 hours before all samples were collected. The median (range) duration of administration was 60.0 (6-276) hours. Dexmedetomidine concentration ranged from 0.07 to 3.17 ng/ml. Dexmedetomidine concentration was not correlated with the administered dose (r = 0.273, P = 0.007). The approximate linear equation was y = 0.690x + 0.423 (Fig. 2). The RASS score ranged from 0 to –5 (Table 2).
Subgroup analyses indicated that neither single- or two-ventricle repair, RACHS-1 score, nor surgical strategy influenced the relationship between dexmedetomidine dose and plasma concentration (Table 3). There was also no significant correlation when only the data of patients with five or more samples were analyzed (Table 3, Fig. 3). Finally, age did not influence the strength of the relationship between dexmedetomidine dose and its plasma concentration: the correlation coefficient was 0.325 in those aged ≤ 1 year (sample number = 72, P = 0.005), compared with 0.310 in those aged ≥ 1 year but ≤ 2 years (sample number = 24, P = 0.140).
The median [IQR] doses of co-administered drugs were as follows: fentanyl, 1.35 [0.66–2.56] µg/kg/h; morphine, 9.80 [6.00–15.39] µg/kg/h; midazolam, 0.16 [0.10–0.33] mg/kg/h; diazepam, 0.41 [0.38–0.48] mg/kg/day; triclofos, 42.6 [11.0–64.1] mg/kg/day; clonidine, 13.2 [9.90–16.0] µg/kg/day; and phenobarbital, 2.94 [2.94–2.94] mg/kg/day.
We found that plasma dexmedetomidine concentration ranged from 0.07 to 3.17 ng/ml in critically ill children under 2 years old administered a dose of 0.2–1.40 µg/kg/h. There was no relationship between plasma dexmedetomidine concentration and the administered dose, even in specific subgroups, in contrast to our previous study in adults that identified a moderate correlation [15]. In our cohort of critically ill infants, administration of the recommended dose of dexmedetomidine did not reliably achieve an optimal plasma concentration. A recent systematic review reported that while 57.6% of PICU patients were optimally sedated, 10.6% were under-sedated and 31.8% were over-sedated [19]. Our findings may explain the high proportion of PICU patients that are not optimally sedated. Physicians must therefore take great care when administering dexmedetomidine to infants.
Although the sedative concentration of dexmedetomidine in infants is not known, the concentrations that we identified were judged to be sufficient to achieve sedation in our cohort. The range in our cohort corresponded closely to the sedative concentration reported in adults (0.2–3.2 ng/ml) [20]. Chrysostomou et al. [21] reported that after cardiac surgery in neonates and infants, a higher maximum dexmedetomidine dose of 1.25 µg/kg/h was well tolerated and provided adequate sedation. In addition, Vilo et al. [22] noted a trend toward a need for an increased dexmedetomidine dose in patients younger than 1 year old. Furthermore, when dexmedetomidine was administered to our cohort with other analgesics and sedatives, we achieved RASS scores from 0 to −5 and only 9.4% had scores of −4 or −5, which are suggestive of over-sedation. Based on these findings, our dexmedetomidine concentration range (0.07–3.17 ng/ml) is likely to represent the sedative concentration in infants.
There may be several explanations for the absence of a relationship between dexmedetomidine dose and its plasma concentration in the present study compared to our previous finding of a moderate correlation in adults, some of which may be a consequence of the study's limitations. First, dosing regimens were based on body weight, a greater proportion of which may change during critical illness in infants compared with adults (all participants in our cohort weighed < 10 kg). Furthermore, body weight was measured before surgery or the onset of critical illness. Changes in body weight, and concomitant changes in blood volume, may be responsible for the variation in plasma dexmedetomidine concentration found here. Second, the pharmacokinetics and pharmacodynamics of dexmedetomidine are poorly understood and unpredictable in this population, particularly in infants with organ failure [23], and both may differ substantially from those in critically ill adults due to differences in protein binding, distribution, and clearance [24]. Third, our cohort was recruited from a single center and all had undergone cardiac surgery, which was performed during the same admission in the majority of cases. This population may therefore not completely reflect typical PICU patients with regard to hemodynamic status, disease severity, or neurodevelopmental stage. Infants with normal cardiac anatomy may exhibit a different relationship between dexmedetomidine concentration and dose. Although a high proportion of participants in our previous study in adults had also undergone cardiac surgery [15], the nature of cardiac disease and comorbidities are substantially different in infants. Fourth, the sample sizes in our subgroup analyses may have been too small to reliably detect a significant relationship between dexmedetomidine dose and its plasma concentration. Future studies should include larger samples of participants with specific cardiovascular disorders, and participants with non-cardiac diseases. Furthermore, as dexmedetomidine is increasingly used to sedate children outside the PICU [2526], it will be important to determine its pharmacodynamic and pharmacokinetic properties in other clinical settings.
Our study had several other limitations. First, as its design was observational, strategies for the administration of dexmedetomidine and other sedative and analgesic drugs were tailored to each infant at the discretion of the supervising physician and other clinical staff. In future studies, consideration should be given to administering other sedatives and analgesics according to a protocol, titrated by sedation and pain scores. Second, multiple samples were obtained from some patients, so we cannot exclude the possibility that these may have influenced our findings for all participants. Finally, we defined an infant as a child under 2 years old, when typically the term is used to refer to children under 1 year old. Unlike some institutions where the youngest children are admitted to a neonatal intensive care unit, we admit all critically ill children to our PICU from birth onward. Consequently, including patients up to the age of 2 years more closely represents our routine clinical practice; furthermore, we found that age did not influence the correlation coefficient.
In conclusion, a dexmedetomidine dose of 0.12–1.40 µg/kg/h achieved a sedative plasma dexmedetomidine concentration of 0.07–3.17 ng/ml in critically ill infants under 2 years old. There was no relationship between dexmedetomidine dose and its plasma concentration. These observations suggest that administration of the recommended dose of dexmedetomidine did not reliably achieve the optimal plasma concentration in critically ill infants.
Acknowledgments
The authors thank Dr. Yoshiki Sento, Dr. Shinichiro Yoshimura, Dr. Yoshiyuki Nishizawa, Dr. Emi Sato, Dr. Miki Nakano, Dr. Kazuma Fujikake, Dr. Satoshi Aoki, Dr. Yukiko Mori, Dr. Taiki Kojima, Dr. Kentaro Miyake, and Dr. Toshihiro Yasui for their help collecting arterial samples and acquiring the data.
References
1. Krauss B, Green SM. Procedural sedation and analgesia in children. Lancet. 2006; 367:766–780. PMID: 16517277.

2. Tobias JD. Dexmedetomidine: applications in pediatric critical care and pediatric anesthesiology. Pediatr Crit Care Med. 2007; 8:115–131. PMID: 17273114.

3. Hosokawa K, Shime N, Kato Y, Taniguchi A, Maeda Y, Miyazaki T, et al. Dexmedetomidine sedation in children after cardiac surgery. Pediatr Crit Care Med. 2010; 11:39–43. PMID: 19593247.

4. Tokuhira N, Atagi K, Shimaoka H, Ujiro A, Otsuka Y, Ramsay M. Dexmedetomidine sedation for pediatric post-Fontan procedure patients. Pediatr Crit Care Med. 2009; 10:207–212. PMID: 19188869.

5. Gupta P, Whiteside W, Sabati A, Tesoro TM, Gossett JM, Tobias JD, et al. Safety and efficacy of prolonged dexmedetomidine use in critically ill children with heart disease. Pediatr Crit Care Med. 2012; 13:660–666. PMID: 22791093.

6. Lam F, Bhutta AT, Tobias JD, Gossett JM, Morales L, Gupta P. Hemodynamic effects of dexmedetomidine in critically ill neonates and infants with heart disease. Pediatr Cardiol. 2012; 33:1069–1077. PMID: 22327182.

7. Czaja AS, Zimmerman JJ. The use of dexmedetomidine in critically ill children. Pediatr Crit Care Med. 2009; 10:381–386. PMID: 19325505.

8. Le KN, Moffett BS, Ocampo EC, Zaki J, Mossad EB. Impact of dexmedetomidine on early extubation in pediatric cardiac surgical patients. Intensive Care Med. 2011; 37:686–690. PMID: 21308359.

9. Mason KP, Lerman J. Dexmedetomidine in children: current knowledge and future applications. Anesth Analg. 2011; 113:1129–1142. PMID: 21821507.
10. Buck ML. Dexmedetomidine use in pediatric intensive care and procedural sedation. J Pediatr Pharmacol Ther. 2010; 15:17–29. PMID: 22477789.

11. Scheinin H, Aantaa R, Anttila M, Hakola P, Helminen A, Karhuvaara S. Reversal of the sedative and sympatholytic effects of dexmedetomidine with a specific alpha2-adrenoceptor antagonist atipamezole: a pharmacodynamic and kinetic study in healthy volunteers. Anesthesiology. 1998; 89:574–584. PMID: 9743392.
12. Ingersoll-Weng E, Manecke GR Jr, Thistlethwaite PA. Dexmedetomidine and cardiac arrest. Anesthesiology. 2004; 100:738–739. PMID: 15108994.

13. Petroz GC, Sikich N, James M, van Dyk H, Shafer SL, Schily M, et al. A phase I, two-center study of the pharmacokinetics and pharmacodynamics of dexmedetomidine in children. Anesthesiology. 2006; 105:1098–1110. PMID: 17122572.

14. Mahmoud M, Mason KP. Dexmedetomidine: review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br J Anaesth. 2015; 115:171–182. PMID: 26170346.

15. Fujita Y, Inoue K, Sakamoto T, Yoshizawa S, Tomita M, Maeda Y, et al. A comparison between dosages and plasma concentrations of dexmedetomidine in clinically ill patients: a prospective, observational, cohort study in Japan. J Intensive Care. 2013; 1:15. PMID: 25705407.

16. Venn RM, Karol MD, Grounds RM. Pharmacokinetics of dexmedetomidine infusions for sedation of postoperative patients requiring intensive caret. Br J Anaesth. 2002; 88:669–675. PMID: 12067004.
17. Iirola T, Ihmsen H, Laitio R, Kentala E, Aantaa R, Kurvinen JP, et al. Population pharmacokinetics of dexmedetomidine during long-term sedation in intensive care patients. Br J Anaesth. 2012; 108:460–468. PMID: 22277665.

18. Inoue K, Sakamoto T, Fujita Y, Yoshizawa S, Tomita M, Min JZ, et al. Development of a stable isotope dilution UPLC-MS/MS method for quantification of dexmedetomidine in a small amount of human plasma. Biomed Chromatogr. 2013; 27:853–858. PMID: 23401046.

19. Vet NJ, Ista E, de Wildt SN, van Dijk M, Tibboel D, de Hoog M. Optimal sedation in pediatric intensive care patients: a systematic review. Intensive Care Med. 2013; 39:1524–1534. PMID: 23778830.

20. Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000; 93:382–394. PMID: 10910487.

21. Chrysostomou C, Sanchez De Toledo J, Avolio T, Motoa MV, Berry D, Morell VO, et al. Dexmedetomidine use in a pediatric cardiac intensive care unit: can we use it in infants after cardiac surgery? Pediatr Crit Care Med. 2009; 10:654–660. PMID: 19295456.

22. Vilo S, Rautiainen P, Kaisti K, Aantaa R, Scheinin M, Manner T, et al. Pharmacokinetics of intravenous dexmedetomidine in children under 11 yr of age. Br J Anaesth. 2008; 100:697–700. PMID: 18378546.

23. Zuppa AF, Barrett JS. Pharmacokinetics and pharmacodynamics in the critically ill child. Pediatr Clin North Am. 2008; 55:735–755. PMID: 18501763.

24. Vet NJ, de Hoog M, Tibboel D, de Wildt SN. The effect of inflammation on drug metabolism: a focus on pediatrics. Drug Discov Today. 2011; 16:435–442. PMID: 21376135.

25. Kim SK, Song MH, Lee IJ, Lee JH, Choi IC. Dexmedetomidine for sedation in pediatric patients who received more than 20 sessions of radiation therapy: two cases report. Korean J Anesthesiol. 2016; 69:627–631. PMID: 27924206.

26. Ko BJ, Jang JH, Park JW, Lee SC, Choi SR. Procedural sedation with dexmedetomidine for pediatric endoscopic retrograde cholangiopancreatography guided stone retraction. Korean J Anesthesiol. 2012; 63:567–568. PMID: 23277822.

Fig. 2
Relationship between dexmedetomidine dose and plasma dexmedetomidine concentration in infants. Plasma dexmedetomidine concentration was weakly correlated with the administered dose (r = 0.273, P = 0.007). The approximate linear equation was y = 0.690x + 0.423.
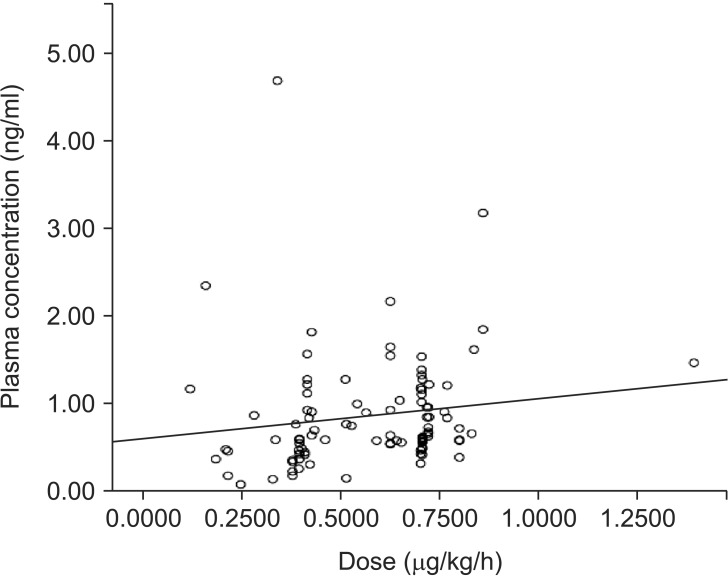
Fig. 3
Subgroup analyses showing the relationship between dexmedetomidine dose and plasma dexmedetomidine concentration in: (A) ventricular septal defect closure; (B, D, and E) curative repair of complete atrioventricular septal defect; (C) curative repair of total anomalous pulmonary venous connection (Table 3); (F) five patients with more than five samples. In (A, B and F) the correlation coefficients were statistically significant; however, there was a wide range of slopes of the approximate linear equations (0.676, 0.826, and 1.729, respectively). It is difficult to definitively conclude that there was a significant correlation between dexmedetomidine dose and plasma dexmedetomidine concentration in these subgroups. The approximate linear equations were: (A) y = 0.826x + 0.031; (B) y = 0.676x + 0.277; (C) y = 1.295x + 0.352; (D) y = 2.544x − 0.543; (E) y = 3.596x − 0.274; (F) y = 1.729x − 0.312.
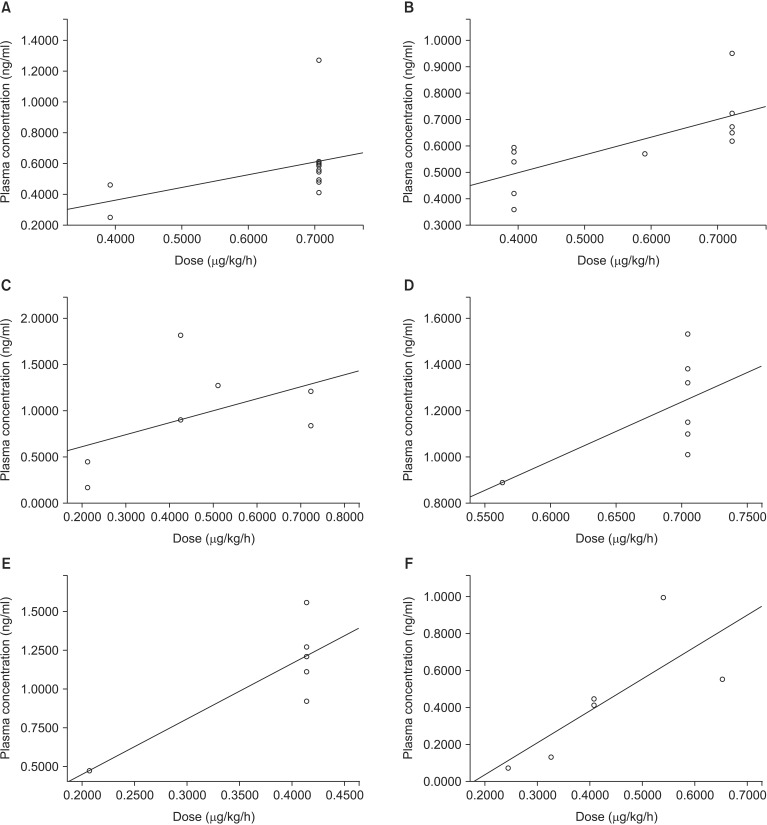
Table 1
Patient Characteristics
Values are mean ± SD (range), median [interquartile range] or number (%).
ICU: intensive care unit, VSD: ventricular septal defect, DORV: double outlet right ventricle, TAPVC: total anomalous pulmonary venous connection, TOF: tetralogy of Fallot, cAVSD: complete atrioventricular septal defect, TGA: transposition of the great arteries, SRV: single right ventricle, SLV: single left ventricle, PDA: patent ductus arteriosus.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download