Abstract
Anesthetic experience in frontotemporal dementia (FTD) with severe hypotension associated autonomic dysfunction has not yet been reported. Here in case, we report on the case of treatment with vasopressin to refractory hypotension in FTD patient. A 54-year-old male presented with a ten-year history of FTD with frequent syncope. The patient was scheduled to undergo subtotal gastrectomy for resection of stomach cancer. During the operation, sudden hypotension occurred and it was refractory to fluid and 1 unit of blood resuscitation and did not respond to catecholamine. Transesophageal echocardiography showed normal heart with adequate volume state. After intravenous administration of arginine vasopressin, the patient's vital signs returned to baseline values. Arginine vasopressin might be considered as a valuable alternative for treatment of severe refractory hypotension in autonomic dysfunction patients with FTD.
Frontotemporal dementia (FTD), characterized by progressive abnormal emotional processing and frontotemporal atrophy, is the third most common cause of neurodegenerative dementia and the second most common in persons younger than 65 years of age [12]. FTD presents with early behavioral abnormalities, including apathy, disinhibition, immoral behavior, obsessive and compulsive behaviors, emotional blunting, and loss of sympathy and empathy.
There have been many reports of frontotemporal dementia related to autonomic dysfunction [34]. Autonomic dysfunction refers as failure or dysregulation of sympathetic noradrenergic innervation [5], which causes neurocardiovascular instability (NCVI) including arterial hypotension and bradycardia.
The orbitofrontal cortex and frontal medial cortex and cerebral amygdala are the relating area for cardiovascular modulation. FTD is characterized by a degenerative process involving many of the anatomical areas above and cardiovascular dysfunction might be more common in these patients with organic dementia like FTD [3].
Although several clinical cases have been reported in various forms, anesthetic experience in FTD with severe autonomic dysfunction has not yet been reported. Here we report on the case of a 54-year-old male presenting with a ten-year history of FTD who developed severe refractory hypotension during general anesthesia. Transesophageal echocardiography (TEE) showed normal valves and contractility with adequate volume state. He did not respond to fluid resuscitation and catecholamine and it was treated successfully with arginine vasopressin. In this case, we assumed that autonomic dysfunction could induce hemodynamic instability even with general anesthesia, moreover vasopressin could be a good choice of treatment in FTD patients with sympathetic noradrenergic failure situations.
A 54-year-old male (height 170 cm, weight 55 kg) with FTD was scheduled to undergo subtotal gastrectomy for treatment of stomach cancer. He was diagnosed with FTD at the age of 44. At the time of diagnosis, he showed apathy and self-destructive impulsivity, such as trying to get out of a moving a car. Recently, he was bedridden most of the time due to weakness of lower limb. In addition, he has experienced frequent syncope and showed fluctuation of blood pressure (BP) without any trigger factor. He did not have special examination for syncope and was not diagnosed as autonomic failure at that time. He had taken anticholinergic drug for the treatment of syncope. His magnetic resonance imaging of the brain showed severe atrophy of bilateral frontal and temporal lobes. The patient has taken selective serotonin reuptake inhibitors, anticonvulsants, dopamine agonist as well as anticholinergic medications.
Preoperative laboratory results were within normal limits, except anemia caused by bleeding of stomach cancer (hemoglobin 8.3 g/dl). Atrial fibrillation was seen on electrocardiogram. The patient and his family had no notable history related to anesthesia and drugs.
He was transferred to the operating room and standard monitors were applied. Initial vital signs showed BP 110/85 mmHg, heart rate (HR) 85 beats/min.
Anesthesia was administered intravenously with thiopental 170 mg and vecuronium 6 mg and intubated successfully. Mechanical ventilation was started with a tidal volume of 450 ml and a respiration rate of 10 breaths/min under FIO2 0.5, maintaining end tidal CO2 as 30–35 mmHg.
Anesthesia was maintained with sevoflurane and vital signs were stable, with systolic/diastolic BP 110–130/70–90 mmHg and HR 80–95 beats/min.
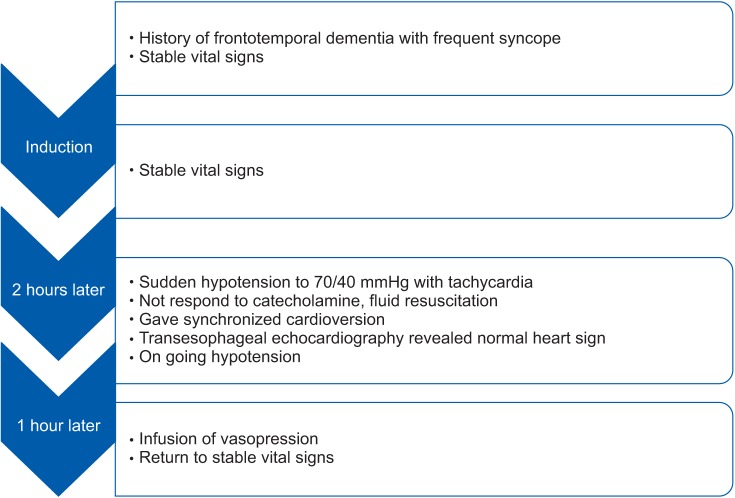
Two hours after induction, the patient showed a sudden drop in BP to 70/40 mmHg. There was no significant event, except shedding 300 ml of blood. The electrocardiogram showed atrial fibrillation at varying rates between 110–130 beats/min. We considered that tachycardia would be the cause of sudden hypotension because we administered volume of 1,500 ml of Lactated Ringer's solution and 1 unit of packed red blood cells for compensating the blood loss until that time. We immediately consulted with the cardiologist and TEE was performed. TEE showed normal valves and contractility with adequate volume state and no regional wall motion abnormality (estimated ejection fraction was 65%). HR was returned to 50 beats/min after synchronized cardioversion at 50 J, however, no change in BP was observed. The patient's vital signs showed no response at bolus injection of ephedrine, we infused dopamine of 10 µg/kg/min, dobutamine of 10 µg/kg/min, and norepinephrine of 0.3 µg/kg/min. There was no response to those drugs. Only high dose of epinephrine at 0.5–1.0 mg induced increasing BP. Thus, we decided to use vasopressin at a rate of 4 units/h after 10 units of bolus dose. The patient's BP and HR then returned to baseline values (BP 110/65 mmHg; HR 75 beats/min) and their stability was maintained. We infused dopamine of 5 µg/kg/min with dobutamine of 5 µg/kg/min and arginine vasopressin at a rate of 4 U/h after resuscitation.
The operation was completed successfully, and the patient was transferred to the intensive care unit. All vasopressors were titrated in order to maintain vital signs and stopped completely at 24 h after surgery, and the patient was extubated. Two days after the operation, he was transferred to the general ward without any sequelae. Information from this case is described into a timeline (Fig. 1).
Frontotemporal lobar degeneration (FTLD) is a common cause of presenile dementia. FTLD, formerly called Pick's disease, is a progressive disease associated with focal atrophy of the frontal and/or temporal lobes. Patients with FTD are often related to autonomic dysfunction [4].
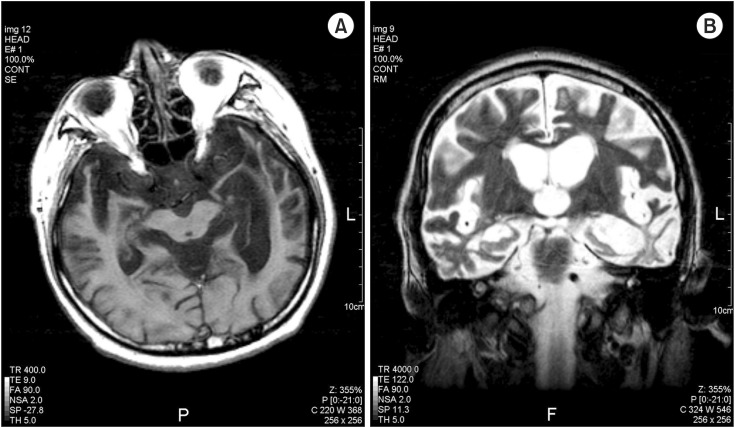
Various areas of cerebral cortex and certain subcortical subnuclei participate in the neural circuits that influence cardiovascular activity. The orbitofrontal cortex, frontal medial cortex including the anterior cingulate, the insula and cerebral amygdale are involved in cardiovascular modulation. Especially, the insular cortex incorporates many functions and the ventral anterior insular is an important autonomic cortical region. In FTD, it targets these systems, and leading to autonomic dysfunction including bradycardia or hypotension [4]. Fig. 2 shows the brain MRI examination of our case patient, which revealed FTD involving various area mentioned above.
The autonomic nervous system contains at least five components; enteric, parasympathetic cholinergic, sympathetic cholinergic, sympathetic noradrenergic, and adrenomedullary hormonal [6]. FTD with autonomic dysfunction patients exhibits one or more failure of these components. Among these components, sympathetic noradrenergic failure presents as orthostatic intolerance and orthostatic hypotension. Orthostatic hypotension may not be evident in the operating room, anesthetic management worsens cardiovascular function.
Surgical blood loss disturbs the maintenance of baroreflex control and anesthetic agents can suppress the residual capacity to release catecholamines and induce diminished baroreflex gain. That results in further compromises the pressor response during surgery, this is more exacerbated due to their inability to buffer changes in BP [7].
In this case, the patient had suffered from frequent syncope preoperatively. That suggests he had NCVI in the form of autonomic dysfunction with regards to FTD. NCVI is defined as “age-related changes in BP and heart rate behavior, predominantly resulting in hypotension and bradyarrhythmia.” The most common manifestations of NCVI are carotid sinus syndrome, orthostatic hypotension, postprandial hypotension, and vasovagal syndrome, and clinical findings are similar to those of catecholamine-resistance vasoplegic shock.
The loss of cardiovascular reflexes in autonomic failure can complicate anesthesia and lead to life threatening changes in BP. The volume status of the patients is one of the most important factors in determining hemodynamic stability in autonomic failure patients. Acute changes in blood volume can result in a substantial rise or fall in BP [8].
In this case, the patients had autonomic dysfunction with FTD, after shedding of 300 ml of blood, it would trigger NCVI and caused severe hypotension. Furthermore, echocardiography revealed normal heart function during hypotensive event, we could exclude heart failure from any other causes or ischemic heart disease.
Mesenteric traction syndrome could be another cause of hypotension during upper abdominal surgery, which is characterized by a triad of hypotension, tachycardia, and flushed skin [9]. It usually occurs in the early phase of laparotomy, when the surgeon begins abdominal exploration and traction of the small intestine. Fluid replacement, vasopressors, and nonsteroidal anti-inflammatory drugs could be used as a treatment. In our case, the hypotensive event had occurred 2 hours later after operation which was quite later than usual cases and there was no skin flushed symptom, thus we could exclude mesenteric traction syndrome from cause of refractory hypotension.
Vasopressin is a peptide synthesized in the hypothalamus, and its primary role is fluid homeostasis, moreover, is has been recognized as an adjunct treatment for septic and vasoplegic shock. Vasopressin restores vascular tone in vasoplegic (catecholamine-resistant) shock states by at least four known mechanisms [10], through activation of V1 vascular receptors; modulation of ATP-sensitive K+ channels; modulation of nitric oxide; and potentiation of adrenergic and other vasoconstrictor agents. Prolonged shock is associated with a drop in vasopressin levels, probably due to depletion of vasopressin stores [101112], and may contribute to the refractory hypotension seen in advanced shock states.
Vasopressin release is under the control of the autonomic nervous system. Primary autonomic failure is associated with hyposensitivity to the pressor effects of vasopressin as well as with abnormalities of its release [13]. NCVI in FTD is the end result of autonomic nervous system dysfunction, suggesting that FTD patients were likely to be in vasopressin depletion states and it implicates sympathetic adrenergic dysregulation or failure. In the condition of sympathetic adrenergic dysregulation, the response to various cardiovascular vasodepressor drugs maybe exaggerated, while the response to catecholamine would be unpredictable. Vasopressin also produces direct smooth muscle vasoconstriction independent of adrenergic response [14].
Catecholamine, due to its mechanism of action that includes release of norepinephrine from the postganglionic neurons, these agents might only be effective in patients with residual sympathetic activity and capacity to release norepinephrine. This is why ephedrine, dopamine and dobutamine were not effective in this case.
A few cases reports have described the successful administration of vasopressin, and reported rapid hemodynamic stabilization after bolus injection of vasopressin (2–10 units) [1315]. In one report, injection of vasopressin 10 units was followed by a continuous infusion of vasopressin (40 units over 60 min). The current patient responded to a bolus of vasopressin 10 units, followed by a continuous infusion at a rate of 4 units/h. The use of low-dose or high dose vasopressin infusions has become an accepted alternative for management of vasoplegic shock refractory to catecholamine.
It is important to remember that autonomic dysfunction with FTD can present as a catecholamine-refractory shock, such as that demonstrated by our case. In addition, autonomic dysfunction should be considered as a cause of sudden collapse during anesthesia and appropriate treatment should be initiated quickly in order to maintain adequate cerebral perfusion.
In conclusion, FTD is related to severe hypotension due to autonomic dysfunction. In anesthesia of patients with FTD, arginine vasopressin might be considered as a valuable alternative for treatment of severe hypotension during anesthesia.
Acknowledgments
This work was supported by clinical research grant from Pusan National University Hospital.
References
1. Ballard C, Shaw F, McKeith I, Kenny R. High prevalence of neurovascular instability in neurodegenerative dementias. Neurology. 1998; 51:1760–1762. PMID: 9855544.

2. Gustafson L, Brun A, Passant U. Frontal lobe degeneration of non-Alzheimer type. Baillieres Clin Neurol. 1992; 1:559–582. PMID: 1344203.
3. Robles Bayón A, Gude Sampedro F, Torregrosa Quesada JM. Bradycardia in frontotemporal dementia. Neurologia. 2014; 29:76–85. PMID: 23601757.

4. Struhal W, Javor A, Brunner C, Benesch T, Schmidt V, Vosko MR, et al. The phoenix from the ashes: cardiovascular autonomic dysfunction in behavioral variant of frontotemporal dementia. J Alzheimers Dis. 2014; 42:1041–1046. PMID: 25024313.

5. Goldstein DS, Robertson D, Esler M, Straus SE, Eisenhofer G. Dysautonomias: clinical disorders of the autonomic nervous system. Ann Intern Med. 2002; 137:753–763. PMID: 12416949.

6. Goldstein DS. Dysautonomia in Parkinson's disease: neurocardiological abnormalities. Lancet Neurol. 2003; 2:669–676. PMID: 14572735.

7. Mustafa HI, Fessel JP, Barwise J, Shannon JR, Raj SR, Diedrich A, et al. Dysautonomia: perioperative implications. Anesthesiology. 2012; 116:205–215. PMID: 22143168.
8. Bannister R, Ardill L, Fentem P. Defective autonomic control of blood vessels in idiopathic orthostatic hypotension. Brain. 1967; 90:725–746. PMID: 6075807.

9. Takahashi H, Shida D, Tagawa K, Suzuki T. Hemodynamics of mesenteric traction syndrome measured by FloTrac sensor. J Clin Anesth. 2016; 30:46–50. PMID: 27041263.

10. Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med. 2001; 345:588–595. PMID: 11529214.

11. Landry DW, Levin HR, Gallant EM, Ashton RC Jr, Seo S, D'Alessandro D, et al. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997; 95:1122–1125. PMID: 9054839.

12. Sharshar T, Carlier R, Blanchard A, Feydy A, Gray F, Paillard M, et al. Depletion of neurohypophyseal content of vasopressin in septic shock. Crit Care Med. 2002; 30:497–500. PMID: 11990905.

13. Möhring J, Glänzer K, Maciel JA Jr, Düsing R, Kramer HJ, Arbogast R, et al. Greatly enhanced pressor response to antidiuretic hormone in patients with impaired cardiovascular reflexes due to idiopathic orthostatic hypotension. J Cardiovasc Pharmacol. 1980; 2:367–376. PMID: 6156335.

14. Stoelting RK. Hormones as drugs. Pharmacology and Physiology in Anesthetic Practice. 3rd ed. Philadelphia: Lippincott-Raven;1999. p. 423.
15. Williams SR, Denault AY, Pellerin M, Martineau R. Vasopressin for treatment of shock following aprotinin administration. Can J Anaesth. 2004; 51:169–172. PMID: 14766695.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download