Introduction
Eclampsia is most common complication of pregnancy with hypertension and proteinuria, with an incidence of 6–8% [
1]. Magnesium sulfate (MgSO
4) is used widely for the treatment and prevention of seizures in patients with eclampsia [
2]. In the clinical field, to treat eclampsia, MgSO
4 is initially bolus injected as a loading dose with 4 g and 2 g/h is infused as maintenance dose, such that the serum concentration of magnesium reaches 2.0–3.5 mmol/L. MgSO
4 enhances the effects of muscle relaxation on non-depolarizing neuromuscular blocking agents (NMBA) by depressing the acetylcholine release at the motor end plate within neuromuscular junction, decreasing the excitability of muscular fibers, and decreasing the electric potential amplitude at the end plate [
3].
Sugammadex, a modified γ-cyclodextrin, is a selective relaxant-binding agent. Sugammadex achieves rapid reversal of muscle relaxation by forming a tight complex with unbound steroidal NMBA molecules, such as rocuronium or vecuronium. A deep neuromuscular block (NMB) by rocuronium (1–2 post-tetanic counts [PTC]) is reversed with 4.0 mg/kg of sugammadex [
4]. A deep NMB has various advantages. It can provide a better surgical field with lower intra-abdominal pressure than a moderate NMB (1–2 train of four [TOF] counts), decrease postoperative pain and induce rapid recovery of bowel function [
5]. It also improves the surgical view in microsurgery [
6]. Czarnetzki et al. [
7] reported that there was no effect of MgSO4 pretreatment on the sugammadex reversal for a deep NMB with rocuronium. However, this study was performed with one bolus injection of 60 mg/kg MgSO
4 and the serum magnesium concentration was 1.5 mmol/L; this concentration was lower than the clinical concentration of 2.0–3.5 mmol/L for eclampsia treatment. In addition, there were various limitations for the maintenance of constant serum concentrations of magnesium.
The aim of this study was to evaluate the effect of high serum magnesium concentration on the sugammadex reversal of a rocuronium-induced deep NMB in experimental animals.
Materials and Methods
After approval by our institutional animal care and use committee (CLAS MRCC HYU 2014-0069), we used 28 adult male New Zealand white rabbits, weighing 3.3–3.8 kg (3.5 ± 0.2 kg). The animals were anesthetized with 100% oxygen (oxygen flow 200 ml/kg/min) and sevoflurane (Sevoflo® Abbott Laboratories Ltd, Kent, UK) using a face mask attached to a Bain circuit for the induction. Tracheal intubation with a 3.0 uncuffed tube was performed using a blind technique with the guidance of capnography [
8] and the lungs were ventilated with an animal respirator (SN-480-5, Shinano Co., Ueda City, Japan). Ventilation was controlled to maintain end-tidal carbon dioxide ranging from 30–35 mmHg, as measured by an ETCO
2/SpO
2 monitor (CO
2SMO®, Novametrix Co., Wakefield, OH, USA), with a tidal volume of 25 ml/kg, a respiratory rate of 30–35 breaths/min and inspiration time : expiration time (I : E) ratio of 1 : 2. To continuously monitor the end-tidal sevoflurane concentration between the intubation tube and the animal respirator, a gas analyzer (Datex-Ohmeda S/5 Anesthesia Monitor; GE Healthcare Finland, Helsinki, Finland) was used. The minimum alveolar concentration of sevoflurane was 3.7 ± 0.16% in rabbits; therefore, the end-tidal concentration of sevoflurane for maintenance of adequate anesthesia was controlled at 4% [
9]. A common carotid artery was cannulated for monitoring arterial blood pressure and intermittent analysis of arterial blood gases. A four-limb electrocardiogram was used for heart rate monitoring.
The rabbits were randomly assigned to four groups (control group [n = 7], 2-mg group [n = 7], 4-mg group [n = 7], and 8-mg group [n = 7]). The position of all animals was supine. The right ear vein was cannulated for intravenous (IV) fluid and drug administration. In the control group, normal saline 5 ml/kg was infused for 20 minutes using a syringe pump 1 hour before the study. In the study groups (2-mg, 4-mg and 8-mg groups), 50% MgSO4 (50 mg/ml) 150–200 mg/kg, which was diluted 10 times with normal saline, was infused using the same method as in the control group, and the maintenance dose of MgSO4 25 mg/kg/h was continuously infused to maintain a constant serum magnesium concentration (2.0–3.5 mmol/L). Drug dosages were determined by the weight of the rabbit. The serum magnesium concentrations were measured before administration of MgSO4 and just before and after the study by sampling from the carotid artery. Arterial blood gas analysis was performed using a GEM-STAT® gas analyzer (Mallinckrodt Co., St. Louis, MO, USA). The rectal temperature of the rabbits was maintained at 38℃ using a thermostat (Blanketrol II, 222, Cincinnati Sub-Zero Co., Cincinnati, OH, USA) and a heat lamp.
The right hind leg was shaved, and a longitudinal incision was made along the anterior leg. Dissection was performed to expose the right tibialis anterior muscle and common peroneal nerve. The common peroneal nerve was stimulated supramaximally at the posterolateral aspect of the knee with a 0.2-ms square-wave stimulus from a peripheral nerve stimulator (Dual-Stim®, Life-Tech Inc, Stafford, USA). TOF stimulation was applied once every 10 s. The tendon of the tibialis anterior was attached to a force displacement transducer (45196A®, San-ei Co., Tokyo, Japan) with the use of 2.0 silk. The twitch response was quantified mechanomyographically with a preload tension (20 g). The mechanomyogram was recorded on a multichannel recorder (Biophysiograph 7748®, San-ei Co., Tokyo, Japan). NMB was quantified by the first twitch (T1) of the TOF and TOF ratio (T4/T1).
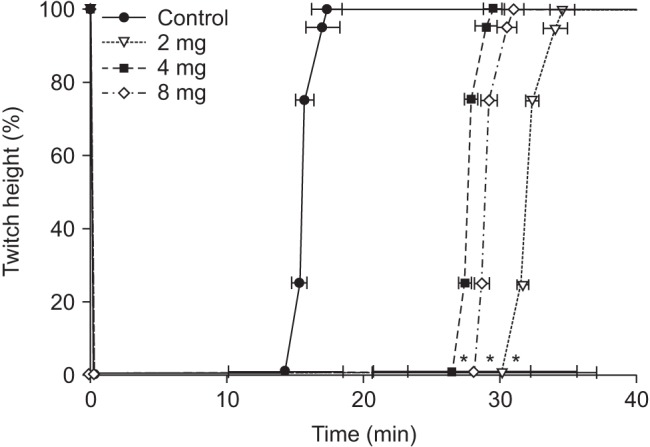
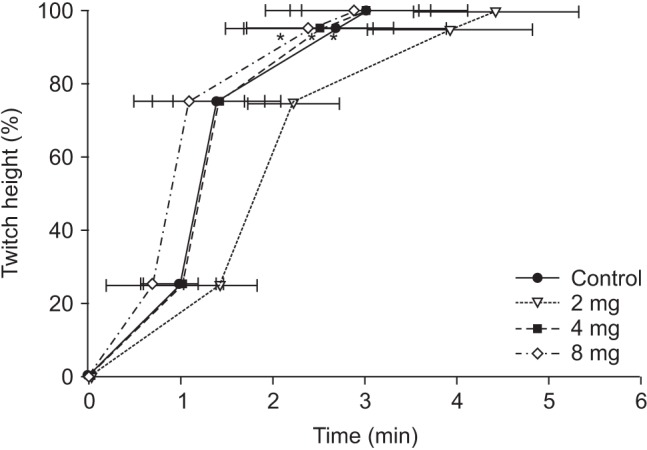
After a stable recording of neuromuscular transmission had been established for 20 min, rabbits in all groups received rocuronium 0.6 mg/kg. After the complete depression of twitch on TOF, PTC was measured every 6 min. When PTC 1–2 appeared, sugammadex 4 mg/kg was administered as reversal agent in the control group and the 4-mg group, 2 mg/kg in the 2-mg group and 8 mg/kg in the 8-mg group. The twitch recordings were evaluated for the following variables: time from the end of the injection of rocuronium to maximal twitch suppression (onset); time from the end of the injection of rocuronium to the appearance of PTC 1–2; time from the end of injection of rocuronium to recovery of T1 in the TOF to a value of 25%, 75%, and 95% of control twitch tension (T1 [25, 75, 95]); time from the end of the injection of rocuronium to a TOF ratio of 90% (TOF [90]). At the end of the study, animals were given a lethal dose of pentobarbital and potassium chloride by IV injection.
Statistical analysis
Statistical analyses were conducted using SPSS 17.0 for window (SPSS Inc., Chicago, IL, USA). To determine the sample size, the technique used in a report was employed [
10]. The mean onset of rocuronioum was 0.5 ± 0.1 min in rabbits. A minimum detected difference of 20% was considered significant. A sample size of 7 in each group was calculated to be appropriate to achieve a power of 0.8 and an α value of 0.05. One-way analysis of variance with
post hoc Bonferroni correction was performed for multiple comparisons among groups. Differences were considered statistically significant at P < 0.05. All variables were expressed as mean ± SD.
Discussion
In this study, more than 590 mg of MgSO4 was required to maintain a serum magnesium concentration above 2.0 mmol/L. The onset time of rocuronium in the study groups was significantly shorter than in the control group and the time for appearance of PTC 1–2 in the study groups was significantly longer than in the control group. In addition, TOF [90], T1 [75] and T1 [95] from the end of sugammadex injection in the 2-mg group were significantly extended relative to those in the other groups.
The normal range of serum magnesium concentration is 0.7–0.9 mmol/L in humans [
11] and 0.5–0.8 mmol/L in rabbits [
12]. The serum magnesium concentration in this study was 0.52–0.60 mmol/L. Previous studies [
7,
131415] found interactions between muscle relaxants and MgSO
4 40–60 mg/kg, which was single IV bolus administered. Therefore, these conditions were very different from eclampsia treatment, which requires a sustained serum magnesium concentration (2.0–3.5 mmol/L) with a continuous infusion of MgSO
4. Even in the study with humans, serum magnesium concentrations were not maintained at the level necessary to treat eclampsia [
16]. In the current study, the loading dose of MgSO
4 150–200 mg/kg was administered for 20 min using a syringe pump before study, and a maintenance dose of MgSO
4 25 mg/kg/h was continuously administered to resemble the clinical situation of eclampsia treatment, maintaining a constant serum magnesium concentration of 2.0–3.5 mmol/L. Therefore, the effect of serum magnesium at the therapeutic concentration for eclampsia on the reversal of sugammadex could be evaluated.
An increase in serum magnesium concentration can induce hypotension. It is the results which magnesium directly antagonizes the calcium effect and acts to vascular smooth muscle and induces vasodilation. Indirectly, magnesium induces a sympathetic block and decreases vasomotor reflex and blood retention of peripheral veins, due to skeletal muscle relaxation. Finally, magnesium decreases venous return to the left ventricle and cardiac output [
17]. However, in this study, there were no differences in blood pressure or heart rate between groups, regardless of sugammadex administration. The reason for this finding was not clear, but might be associated with the greater hemodynamic effect of an inhalational anesthetic agent other than magnesium.
In rabbits, the effective dose resulting in a 50% reduction of <twitch tension (ED
50) and the effective dose resulting in a 95% reduction of twitch tension (ED
95) are 61.5 ± 5.3 µg/kg and 95.1 ± 6.7 µg/kg, respectively [
18]. Kim et al. [
19] reported that the ED
50 value of rocuronium for rabbits was 56.5 mg/kg, a level that was 40% of the level reported for humans [
20]. Therefore, the dose of rocuronium was 0.6 mg/kg was used for a deep NMB, which is the same dose used for intubation in humans. MgSO4 administered 15 min before propofol anesthesia reduces the onset time of rocuronium by approximately 35% and prolongs the total recovery time by approximately 25% [
7]. In the current study, the onset time of rocuronium 0.6 mg/kg in the study groups was significantly shorter than in the control group. This decrease in onset time might be associated with serum magnesium concentrations above 2.0 mmol/L. The onset time of rocuronium 0.6 mg/kg in humans is approximately 1.5 min [
21]. The decreased onset time in rabbits might be due to the increased dose (7 × ED
95) used in rabbits compared with the human dose (2 × ED
95). The longer time (26–30 min) for the appearance of PTC 1–2 in the study groups compared to the control group (14 min) also might be associated with serum magnesium concentration levels. Clinically, the presence or absence of MgSO
4 may have an effect on the emergent reversal with sugammadex after muscle relaxant.
The recommended dose of sugammadex is 2 mg/kg for a moderate NMB, which is determined by a TOF count above 2 twitches, 4 mg/kg for a deep NMB, which is determined by a PTC below 10, and 16 mg/kg for emergent reversal immediately after NMBA administration [
22]. In this study, the dose of sugammadex for reversal was 4 mg/kg, because PTC was 1–2 when sugammadex was administered. In addition, 2 mg/kg and 8 mg/kg of sugammadex were used to evaluate the efficiency of reversal with MgSO
4 pretreatment.
Sugammadex could reverse a rocuronium-induced NMB in a dose-response manner, even among patients treated with MgSO
4 [
23]. Clinically, there was no effect of MgSO
4 pretreatment on the sugammadex reversal for deep NMB with rocuronium [
7]. In this study, T1 [95] in the control and the 4-mg group, which received identical doses of sugammadex, did not vary. These results meant that the pretreatment MgSO
4 did not affect the reversal of sugammadex under the constant serum magnesium concentration of 2.0–3.5 mmol/L in rabbits. In contrast, there was no difference in T1 [95] between the 4-mg group and the 8-mg group. This might be the result of a ceiling effect of sugammadex, although further study is needed. The prolongation of T1 [95] (3.93 ± 0.93 min) in the 2-mg group might be due to a dose of not a deep NMB but a moderate NMB.
TOF ratio represents a presynaptic receptor block, whereas a post-synaptic receptor block is expressed by a depression in the single twitch response [
24]. In the presence of appropriate concentrations of sugammadex, only a low concentration of free NMBA remains in the neuromuscular junction and will preferentially block post-synaptic acetylcholine receptors. This could explain why, after an optimal dose of sugammadex, the recovery of the TOF ratio to 0.9 precedes the recovery of T1, and why the TOF ratio may be fully recovered when this is not yet the case for T1 [
25]. When antagonizing with classical reversal agents (anticholinesterase drugs) or natural recovery, the time course of recovery from NMBA varies from the case with sugammadex; first, there is the full return of T1 and then recovery of the TOF ratio to 90% [
1026]. In this study, TOF [90] appeared at a time similar to T1 [75], and T1 [95] appeared later than TOF [90]. This might be due to the reversal nature of sugammadex.
One limitation of this study was that anesthetic maintenance was performed with sevoflurane. Inhalational anesthesia is more commonly used in clinical practice than intravenous anesthesia, so we used an inhalational anesthetic agent for anesthesia maintenance. However, although it remains equivocal [
2728], the effect of sevoflurane on the recovery of a neuromuscular blockade by rocuronium cannot be completely ignored. In addition, one report suggested that sugammadex could not be affected by inhalational agents [
29]. Therefore, the effect of sevoflurane might be limited. Another limitation was that we tested only a small number (n = 7 in each condition) of rabbits in order to minimize sacrifice of the animals, based on Min et al. [
10]. If the number of rabbits was increased after a measurement of sample size, significant results may be obtained. Although eclampsia occurs only in women, male animals have undergone in this animal study due to disturbances such as the menstrual cycle.
In conclusion, the reversal of sugammadex from a deep rocuronium-induced NMB during the constant serum magnesium concentration of 2.0–3.5 mmol/L was not affected in rabbits. However, the reversal effect of sugammadex varied depending on the dose.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download