This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Subarachnoid block is a widely used technique for cesarean section. To improve the quality of analgesia and prolong the duration of analgesia, addition of intrathecal opioids to local anesthetics has been encouraged. We compared the effects of sufentanil 2.5 µg and 5 µg, which were added to intrathecal hyperbaric bupivacaine.
Methods
We enrolled 105 full term parturients were randomly divided into 3 groups: Group 1 (control), Group 2 (sufentanil 2.5 µg), and Group 3 (sufentanil 5 µg). In every group, 0.5% heavy bupivacaine was added according to the adjusted dose regimen. We determined the maximum level of sensory block and motor block, the quality of intraoperative analgesia, the duration of effective analgesia and side effects.
Results
There were no significant differences among the 3 groups in the maximum level of the sensory block and motor block. Recovery rate of the sensory block, however, was significantly slower in Group 3 than Group 1. Quality of intraopertive analgesia, muscle relaxation, and duration of effective analgesia were enhanced by increasing the dosage of intrathecal sufentanil. Frequencies of hypotension, maximum sedation level, and pruritus were directly related to the dosage of intrathecal sufentanil, whereas nausea and vomiting occurred only in the groups using sufentanil.
Conclusions
The addition of sufentanil 2.5 µg for spinal anesthesia provides adequate intraoperative analgesia and good postoperative analgesia with minimal adverse effects on the mother.
Keywords: Bupivacaine, Cesarean section, Spinal anesthesia, Sufentanil
Introduction
Spinal anesthesia is often used for cesarean section and is favored over epidural anesthesia due to the ease of maneuver, rapid onset, effective sensory and motor block, low failure rate and low systemic toxicity [
1,
2]. Adding an opioid to the intrathecally administered local anesthetic solution is a widely used method for improving the intraoperative and early postoperative quality of the subarachnoid block and reducing intraoperative visceral traction pain [
3,
4].
Morphine is a highly-ionized, hydrophilic opioid, which provides a spinal analgesia of slow onset and long duration, but has side-effects such as nausea, vomiting, pruritus, and late respiratory depression [
5]. On the other hand, the addition of lipophilic opioids, such as fentanyl and sufentanil to local anesthetics, shortens the onset time of the block but produces a lower incidence of side-effects compared with morphine [
2,
6]. Although several reports have shown to compare the effects of adding various opioids to a fixed amount of local anesthetic solution administered intrathecally [
1-
7], there have not been studies that have compared effects of adding various opioids to varying amount 0.5% hyperbaric bupivacaine based on the parturients heights and weights.
The aim of the present investigation was to determine the appropriate amount of sufentanil addition to 0.5% hyperbaric bupivacaine to minimize the side effects and maximize the analgesic effect.
Materials and Methods
The study was performed in a randomized, double-blind fashion after approval by the institutional review board and with the informed written consent of 105 ASA (American Society of Anesthesiologists) physical status I-II women scheduled for elective cesarean section. We excluded parturients who had contraindications to spinal anesthesia or a high risk of gestational hypertension, diabetes and/or placenta previa.
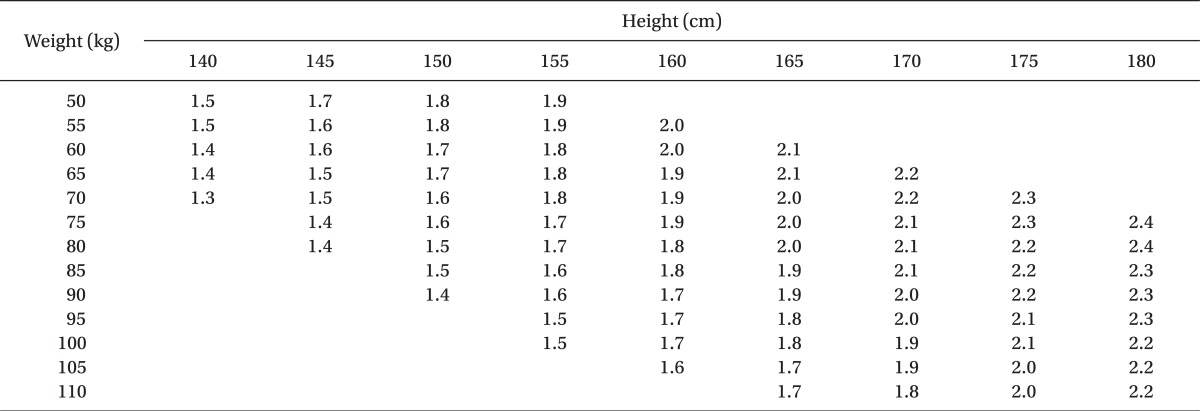
One-hundred-five, healthy term parturients were randomly divided into three groups: Group 1 (control; normal saline 0.2 ml), Group 2 (sufentanil 2.5 µg + normal saline 0.15 ml), Group 3 (sufentanil 5 µg + normal saline 0.1 ml). In every group, 0.5% heavy bupivacaine was added according to the adjusted dose regimen by Harten et al. [
8] (
Table 1). In order to determine sample sizes, power analysis was performed. In respect to the variable of effective analgesia duration the power analysis was done (alpha = 0.05, power = 80%, effect size = 0.5) and 35 parturient were assigned in each group. No subject received premedication and at their arrival into the operating room (OR) they were intravenously injected with 10 ml/kg Ringer's lactate solution via an 18-G venous catheter until the induction of anesthesia. The electrocardiogram (EKG), a non-invasive auto-blood pressure (BP) measurement instrument and pulse oximetry were set up for monitoring the vital signs, while oxygen was provided at 5 L/min using face mask ventilation until the end of the operation.
To perform spinal anesthesia, the patients were placed into a right lateral decubitus position and after the needle insertion site was disinfected, a dural puncture was made with a 26-G Whitacre spinal needle at the L3-L4 or L2-L3 lumbar vertebrae space. The location of the subarachnoid space was then confirmed by the leakage and aspiration of cerebrospinal fluid (CSF). Each hyperbaric solution of bupivacaine (
Table 1) was slowly injected over 30 seconds: there was no addition of opioid for Group 1, sufentanil 2.5 µg was added for Group 2 and sufentanil 5 µg was added for Group 3. After injecting the drug, we put the patients in a supine position and tilted the left side of the operating table down. From the end of injecting the local anesthetics, the patients' BP and pulse were measured at 2 minute intervals during the first 20 min and thereafter at 5 min intervals until the end of the operation. A systolic BP under 90 mmHg or a BP decreased to 20% of the first measured BP was defined as hypotension; when a patient reached this point, they were given 4-8 mg ephedrine promptly infused intravenously. The sensory block levels were evaluated by pinprick tests that were done at 2 min intervals after the induction of anesthesia, while the level of motor blockade was assessed using the Modified Bromage scale; movements are recorded on a 4-point scale: 0 = able to raise an extended leg was no motor block; 1 = unable to raise an extended leg, but able to flex the knee; 2 = unable to flex the knee, but with free movement of the ankle; and 3 = unable to flex the ankle. The maximum level of sensory block and motor block, the sensory block levels and the Bromage scale 120 min after the induction of anesthesia were measured as well. We recorded the side-effects that the patients complained of such as nausea, vomiting, pruritus and/or shivering.
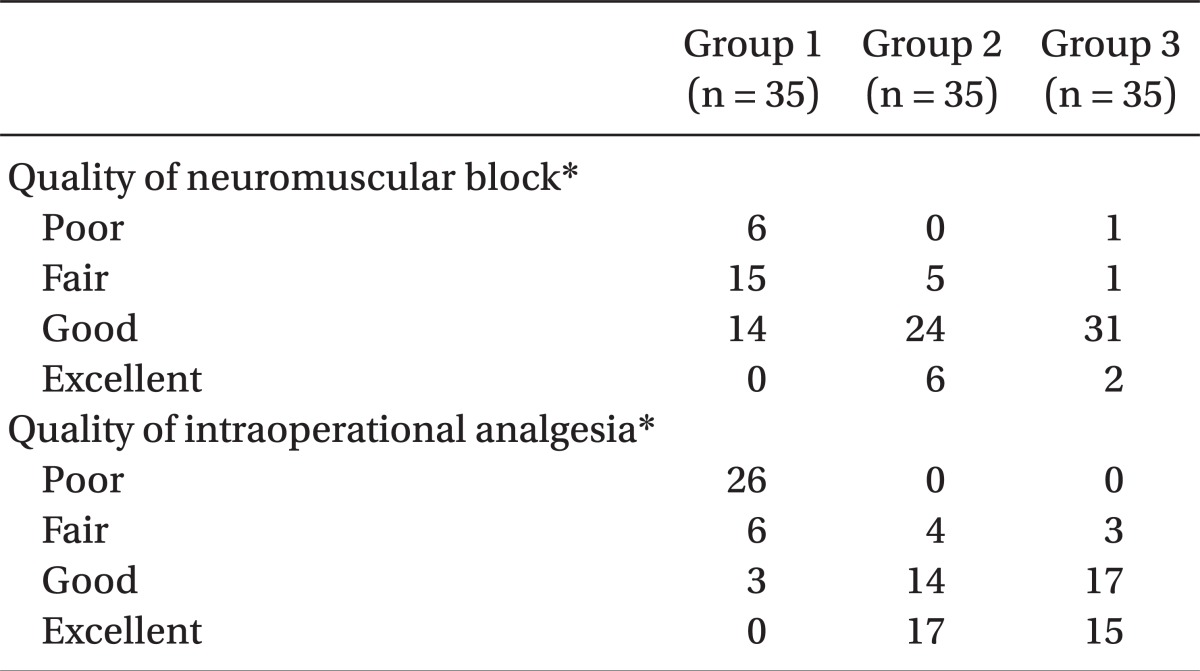
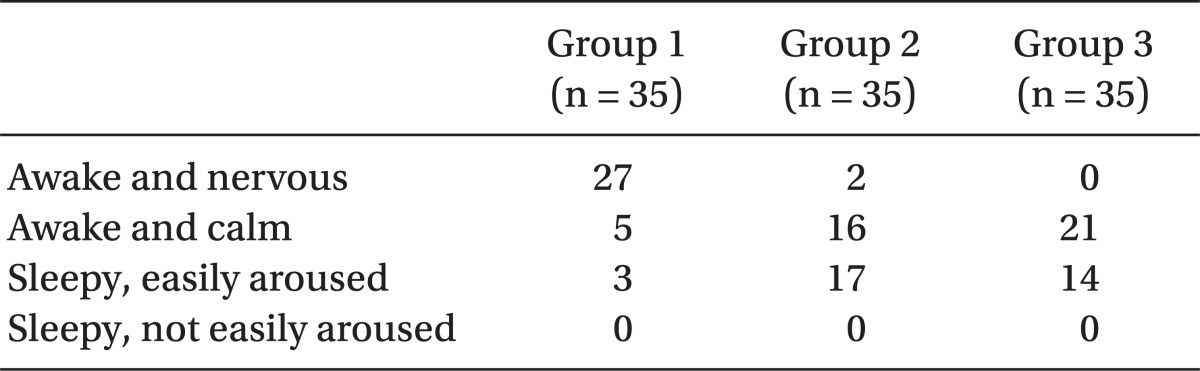
The operation began only when the sensory block level was at level T6 or above. The degrees of muscle relaxation during the operation, which the surgeons rated, were classified into one of 4 grades: 1 = poor, 2 = fair, 3 = good and 4 = excellent. The intraoperative analgesic effects were estimated as 4 grades: excellent = the patient felt comfortable during operation, no complaints; good = a little discomfort, however no need for additive medication; fair = discomfort, but controlled by additive medication, such as fentanyl, propofol, midazolam, etc.; poor = unable to be controlled even with additive medication. In the case of poor, sevoflurane was applied by mask to alleviate discomfort of the patient. The degree of intra-operative sedation of the parturients was described as 1 = awake and nervous, 2 = awake and calm, 3 = sleepy but easily aroused and 4 = sleepy and not easily aroused.
The patient was previously informed to connect the patient controlled analgesia (PCA) after the surgery when she feels post-operative pain of 4 points on a visual analogue scale (VAS). The effective duration of analgesic duration was evaluated indirectly by recording the interval time from the time point of completion the injection of local anesthetics to that of connecting the PCA to the patient.
The Apgar scores were recorded at 1 and 5 min after birth to assess the health of the newborn infants.
For statistical comparison of the 3 groups, Chi-square tests and Fischer's exact tests were used for the maximum Bromage scale (the max. B/S), the Bromage scale at 120 min after the induction of anesthesia (B/S 120 min), the side effects (hypotension, nausea, vomiting, pruritus and/or shivering), degree of muscle relaxation, the intra-operative analgesic effects, and the sedation conditions.
All the other continuous variables were tested for normality Test by using Kolmogorov-Smirnov method. ANOVA test was used for the other observed results, and statistically significant differences were confirmed by Tukey test among the multiple comparison methods. For all of the statistical processes, P < 0.05 was considered statistically significant. Of the variables investigated in this study, all the continuous variables satisfied the hypothesis tests for normality.
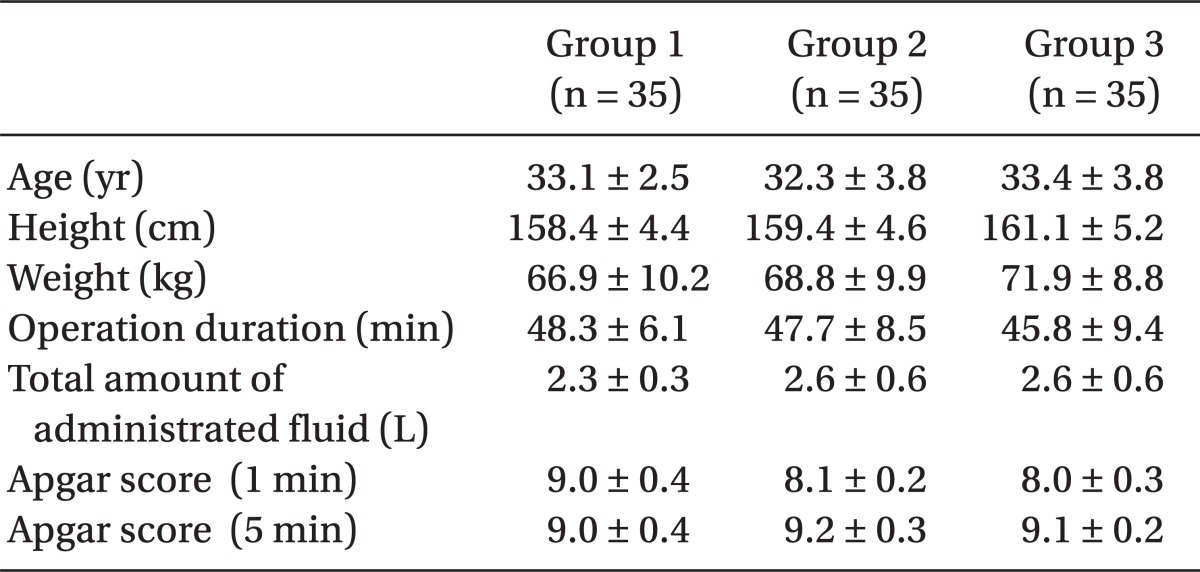
There were no differences between the 3 groups in age, height, weight, or length of operation time, while the mean volumes of bupivacaine used for Group 1, Group 2, and Group 3 were 9.3 ± 0.7, 9.4 ± 0.6 and 9.5 ± 0.7 mg, respectively; and there were no statistically significant differences. In addition, the infused amount of intravenous (IV) fluid, the frequency of hypotension and the dosage of ephedrine did not reveal any statistically significant differences (
Table 2).
Results
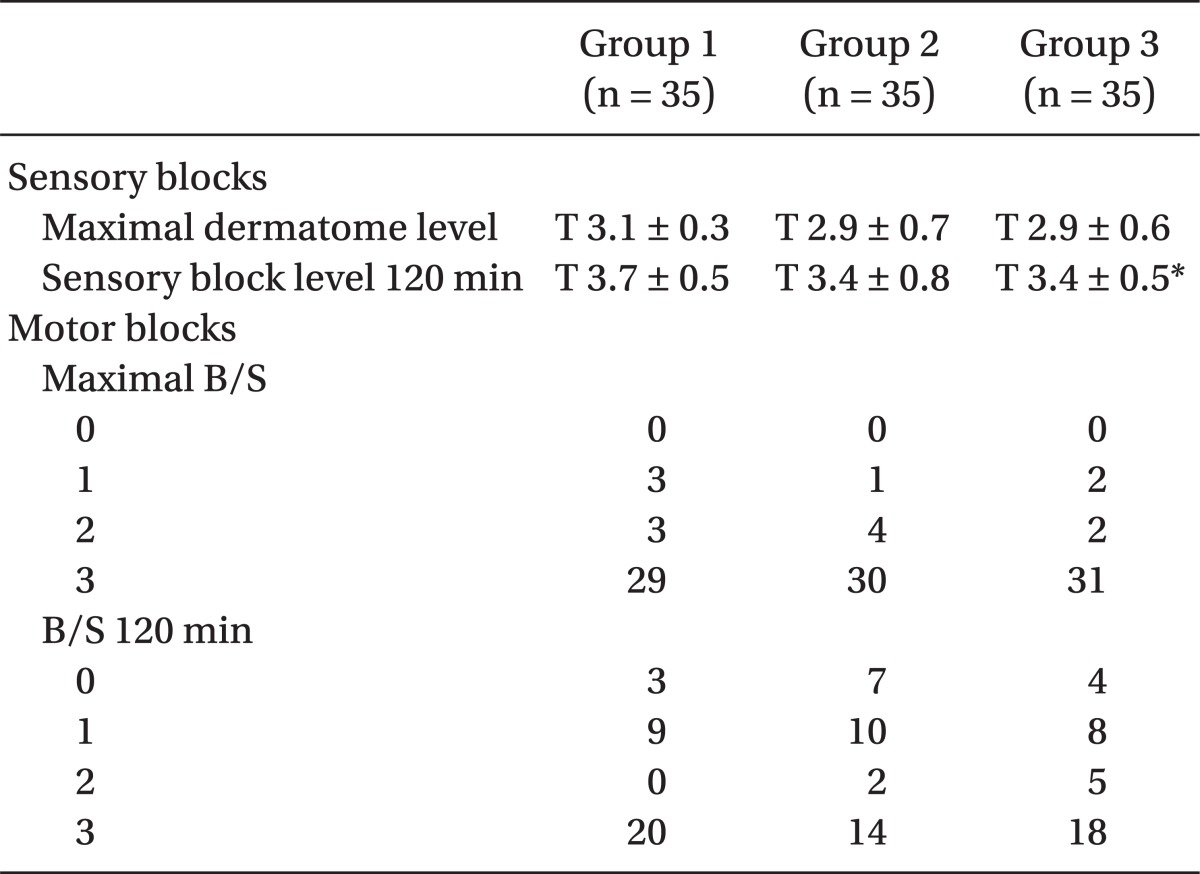
The maximal level of sensory block was attained above T4 in each group; there were no significant differences between the 3 groups, and only the Group 1 and Group 3 had a significant differences in sensory block level at 120 min (P < 0.05). The maximum B/S was a score of 3 for 80% of each group for all 3 groups, and there was no significant difference in response to varying amount of sunfentanil administered (
Table 3).
Intraoperative muscle relaxation and analgesia were enhanced with increasing sufentanil dosage with statistically significance (P < 0.05) (
Table 4). Effective analgesic duration was measured using PCA connecting time and was recorded as means ± SD min. There was a significant, positive correlation (P < 0.05) (ANOVA and Tukey multiple comparison test) between the effective analgesic duration and the amount of sufentanil used as seen among Group 1 (58.0 ± 30.8 min), Group 2 (218 ± 110.9 min), and Group 3 (282.3 ± 110.9 min). The intra-operative sedation of the parturients rose with the increasing amount of sufentanil administered (
Table 5).
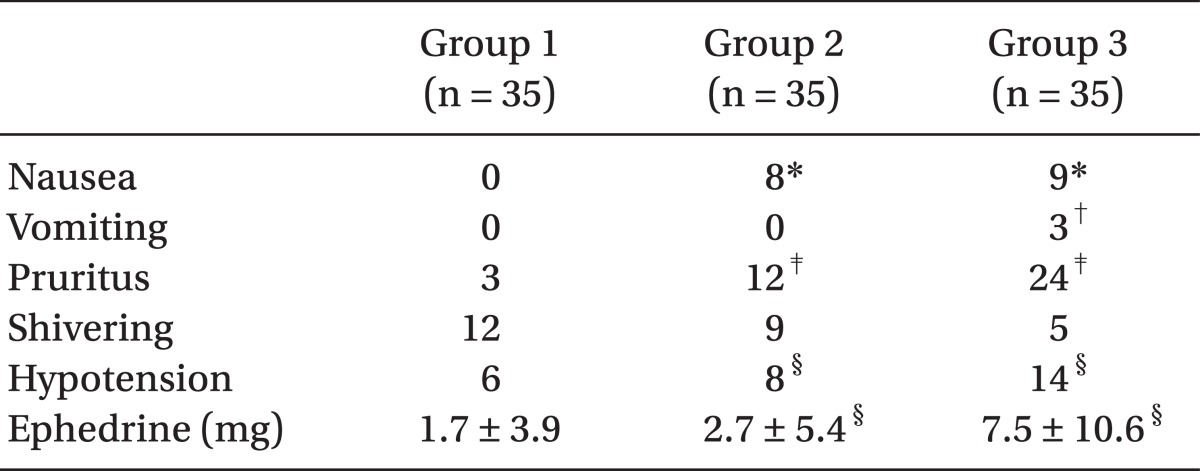
Hypotension frequency also significantly increased with more sufentanil administered and although Group 3 had more ephedrine administered compared to other two groups, there was no significant difference between Group 1 and Group 2. In terms of nausea, the parturients in sufentanil-using Group 2 and 3 experienced more than those in Group 1 but there was no statistical difference between Group 2 and 3 and vomiting was see in only 3 of the Group 3 patients. Pruritus also was more frequent as more sufentanil was administered (
Table 6). There was no significant difference among the three groups in terms of shivering and the Apgar scores measured at 1 and 5 minute after birth were all within normal range and no statistical differences among the groups.
Discussion
Among the anesthesia methods for cesarean section, spinal anesthesia is widely used for its ease of maneuver and fast onset. In addition, the pregnant women can participate in the childbirth experience without risk of the aspiration pneumonia and general anesthesia [
1,
2]. However, the sensory block level may be inadequate and the use of local anesthetics solely may not block the visceral pain adequately due to reasons like abdominal traction [
9]. It is also widely known that hypotension from sympathetic nerve block is frequent.
In order to provide long postoperative analgesia and reduce intra-operative visceral pain, there have been many studies on administration of both local anesthetics and opioids [
10-
15]. Most of these studies have observed that the clinical results with the dose of local anesthetics kept constant, while changing the amount of opioids. Harten et al. [
8] proposed using varying amounts of hyperbaric bupivacaine according to the parturient height and weight, which was reported to reduce the incidence of side effects such as hypotension and more appropriate block level compared to when it was used at a fixed dosage.
Therefore, we compared the clinical effects of adding sufentanil 2.5 µg to 5 µg along with hyperbaric bupivacaine according to the parturient height and weight in order to reduce the side effects and to further enhance the advantages.
Morphine is a highly-ionized, hydrophilic opioid, and is characterized by a slow onset time, because it ionizes readily in the subarachnoid space and binds slowly with the opioid receptors of the spinal cord. Morphine also has a long duration because it remains at a high density in the subarachnoid space, and late respiratory depression may be induced by the rostral spread [
16,
17]. On the other hand, the addition of lipophilic opioids, such as fentanyl and sufentanil to local anesthetics, shortens the onset time of the block but produces a, lower incidence of side-effects compared with morphine [
11].
Wong et al. [
18] reported that more than an addition of 7.5 µg sufentanil revealed more severe pruritus, and a study by Braga et al. [
19] revealed that the incidence of pruritus increases as the dosage of the added sufentanil increases. Dahlgren et al. [
10] reported that when a fixed amount (12.5 mg) of 0.5% hyperbaric bupivacaine with 5.0 µg or 2.5 µg of sufentanil was administered to compare, there was no significant difference in the duration of analgesia, but the pruritus was more frequent when 5 µg was used. Therefore, in order to determine which best maximizes the analgesic effects, lessens the side effects from the increasing amount of local anesthetics, and minimizes the complications from injecting opioids in subarachnoid space, the results from uses of the 2.5 µg or 5.0 µg of sufentanil along with recommended amounts of bupivacaine, according to weight and height, were compared.
There was no difference in the maximum sensory block level, but there was a significant difference in the sensory block level at 120 minutes between Groups 1 and 3. When 5 µg of sufentanil was added, there was a longer duration of sensory block, but motor block and recovery rate did not show any significant difference. In addition, intraoperative and postoperative analgesia was enhanced with increasing amount of sufentanil used.
Braga et al. [
19] reported that the analgesia duration was lengthened significantly for groups that used 5 µg and 7.5 µg of sufentanil compared to control or 2.5 µg of sufentanil group, and Demiraran et al. [
7] reported a similar result as our study, showing a significantly longer analgesic duration for groups that used 1.5 µg, 2.5 µg, and 5.0 µg of sufentanil than that of control group. The present study showed that effective analgesic duration, measured using PCA connecting time, was 218.5 ± 71.8 minutes for 2.5 µg sufentanil, 282.3 ± 110.9 minutes for 5 µg. These results are very different from effective analgesic durations of 12.5 mg bupivacaine with the same amounts of sufentanil, 294 ± 37 min and 346 ± 50 min, of Demiraran et al. [
7], which are most likely because less amounts of bupivacaine were used in the present study.
Braga et al. [
19] reports the higher sedation frequencies in patients with increasing sufentanil dosages and although the present study confirms the significant increase in the sedation with increasing sufentanil dosage, there was no excessive sedation in any patient who did not wake at light arousal. In the present study, although nausea was significantly more frequent in groups that used sufentanil, there was no significant difference between Group 2 and 3. Vomiting was especially more frequent in Group 3 compared to the other 2 groups, which is a similar trend of higher hypotension frequency with increasing sufentanil dosage. Some studies revealed that there was no difference in the hypotension frequency between sufentanil using groups and the control group, but confirmed the higher nausea frequency in the control group [
7,
20]. There were the results from studies that the addition of other kinds opioids than sufentanil cause nausea and hypotension more frequently [
14,
21], and so further studies are required.
The incidence of pruritus with the administration of opioid into the subarachnoid space was reported to be 62% for morphine, 67% for fentanyl, and 80% for sufentanil [
22]. In particular, pruritus due to the addition of opioids in spinal anesthesia may affect the whole body and there is a higher chance of affecting the trigeminal nerves of face and neck regions [
23]. Scott and Fischer [
24] suggest a central encephalinergic mechanism for this localization of pruritus. The spinal nucleus of the trigeminal nerve is rich in opioid receptors and is continuous with the substantia gelatinosa and Lissauer tract at C3-C4.
In the present study, the incidence of pruritus rose with the increase in sufentanil dose, which were 34% and 69% for Group 2 and Group 3, respectively, and although the incidence was about twice higher in Group 3 than Group 2, and the occurrences appeared as mild to the face and upper chest regions. There was no severe case that needed specific treatments, because of pruritus affecting the whole body. There was no significant difference among the three groups in terms of incidence of shivering and the Apgar scores measured at 1 minute and 5 minutes after birth.
In conclusion, intra-operative muscle relaxation and the analgesia were enhanced with increasing dosage of sufentanil. However, although the effective analgesic duration was longer for Group 3 than Group 2, because the vomiting, hypotension, and pruritus frequencies were higher in Group 3 than Group 2, it is recommended to use 2.5 µg of sunfentanil over 5.0 µg with the amounts of 0.5% hyperbaric bupivacine, which varied according to the parturients' height and weight, in order to lower the possible side effects and to lengthen the effective analgesic duration.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download