Abstract
Background
Activation of renin-angiotensin system (RAS) has been an important mechanism of microvascular and macrovascular complications in diabetic patients. It has been reported that RAS blockades reduce the development and progression of diabetic nephropathy. The aim of this study was to evaluate whether valsartan, an angiotensin II receptor blocker (ARB), reduced blood pressure and urinary albumin excretion rate (UAER) in hypertensive type 2 diabetic patients.
Method
Three hundred forty-seven hypertensive type 2 diabetic patients who had not taken angiotensin converting enzyme inhibitors or ARB for 6 months prior to this study were enrolled. We measured blood pressure and UAER before and after 24 weeks of valsartan treatment.
Result
Baseline mean systolic and diastolic blood pressure was 143 ± 15 and 87 ± 11 mmHg, respectively and the median albumin excretion rate was 27 ug/mg. Reduction in systolic and diastolic blood pressure was 16 mmHg/10 mmHg and the median UAER was 19.3 ug/mg after 24 weeks (P < 0.01, respectively). When we divided the subjects into three groups according to the UAER (normoalbuminuria, microalbuminuria and macroalbuminuria), significant changes were reported in the microalbuminuria and the macroalbuminuria groups. Thirty-eight (42%) patients with microalbuminuria improved to normoalbuminuria and twelve (41%) patients with macroalbuminuria improved to microalbuminuria. We found an association between the improvement of blood pressure and UAER (R = 0.165, P = 0.015).
Figures and Tables
Fig. 1
Changes in the urinary albumin excretion rate in each albuminuria group. It was noted that the urinary albumin excretion rate reduced in each group after valsartan (or valsartan/chlorthiazide) administration. The values were reported as median. UAER, urine albumin excretion ratio. *P < 0.05, compared to the initial value.

Fig. 2
Changes of the albuminuria status in each albuminuria group after 24 weeks of valsartan administration. Thirty-eight (43%) patients with microalbuminuria improved to normoalbuminuria and twelve (41%) patients with macroalbuminuria improved to microalbuminuria.
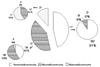
Fig. 3
Correlation between the reduction of systolic blood pressure and urinary albumin excretion rate. Blood pressure reduction was associated with a decreased urinary albumin excretion ratio (R = 0.165, P = 0.015). △SBP, reduction of systolic blood pressure; △UAER, reduction of urinary albumin excretion ratio.

References
1. Mogensen CE, Keane WF, Bennett PH, Jerums G, Parving HH, Passa P, Steffes MW, Striker GE, Viberti GC. Prevention of diabetic renal disease with special reference to micoalbuminuria. Lancet. 1995. 346:1080–1084.
2. Incidence and prevalence of ESRD. Am J Kidney Dis. 1999. 34:S40–S50. USRDS Annual Data Report Chapter 2.
3. Pugh JA, Medina RA, Cornel JC, Basu S. NIDDM is the major cause of diabetic end-stage renal disease: more evidence for a tri-ethnic community. Diabetes. 1995. 44:1375–1380.
4. Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med. 1999. 341:1127–1133.

5. Ruggenenti P, Schieppati A, Remuzzi G. Progression, remission, regression of chronic renal disease. Lancet. 2001. 357:1601–1608.
6. Oliverio MI, Coffman TM. Angiotensin-II receptor: new target for antihypertensive therapy. Clin Cardiol. 1997. 20:3–6.
7. Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Anderson S, Amer P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001. 345:870–878.

8. Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001. 345:851–860.

9. Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001. 345:861–869.

10. Hostetter TH. Prevention of end-stage renal disease due to type 2 diabetes. N Engl J Med. 2001. 345:910–912.

11. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972. 18:499–502.

12. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003. 289:2560–2572.

13. American Diabetes Association. Clinical practice recommendation 2003 - Diabetic nephropathy. Diabetes Care. 2003. 26:94–98.
14. Zandbergen AA, Baggen MG, Lamberts SW, Bootsma AH, de Zeeuw D, Ouwendijk RJ. Effect of losartan on microalbuminuria in normotensive patients with type 2 diabetes mellitus. Ann Intern Med. 2003. 139:90–96.

15. Viberti G, Wheeldon NM. Mircoalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus. Circulation. 2002. 106:672–678.
16. Yusuf S, Ostergren JB, Gerstein HC, Pfeffer MA, Swedberg K, Granger CB, Olofsson B, Probstfield J, McMurray JV. Effects of candesartan on the development of a new diagnosis of diabetes mellitus in patients with heart failure. Circulation. 2005. 112:48–53.

17. Cho MH, Yoo SY, Jung HY, Yoon JE, Nam J, Kim HJ, Park JS, Kim CS, Ha HJ, Gwak HS, Kang ES, Kim HJ, Ahn CW, Cha BS, Song YD, Lim SK, Kim KR, Lee HC. Effect of Candesartan to Plasma MMPs and TIMPs in Hypertensive Type 2 Diabetic Patients. Korean Clinical Diabetes J. 2007. 8:81–91.
18. Ravid M, Brosh D, Levi Z, Bar-Dayan Y, Ravid D, Rachmani R. Use of enalapril to attenuate decline in renal function in normotensive, normoalbuminuric patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1998. 128:982–988.
19. Ahmad J, Siddiqui MA, Ahmad H. Effective postponement of diabetic nephropathy with enalapril in normotensive type 2 diabetic patients with microalbuminuria. Diabetes Care. 1997. 20:1576–1581.

20. Gansevoort RT, de Zeeuw D, de Jong PE. Is the antiproteinuric effect of ACE inhibition mediated by interference in the renin-angiotensin system? Kidney Int. 1994. 45:861–867.
21. Gansevoort RT, de Zeeuw D, de Jong PE. Dissociation between the course of the hemodynamic and antiproteinuric effects of angiotensin I converting enzyme inhibition. Kidney Int. 1993. 44:579–584.

22. Stehouwer CD, Nauta JJ, Zeldenrust GC, Hackerng WH, Donker AJ, Den Ottolander GJ. Urinary albumin excretion, cardiovascular disease and endothelial dysfunction in non-insulin-dependent diabetes mellitus. Lancet. 1992. 340:319–323.

23. Rodrigo E, Maeso R, Munoz-Garcia R, Navarro-Cid J, Ruilope LM, Cachofeiro V, Lahera V. Endothelial dysfunction in spontaneously hypertensive rats; consequences of chronic treatment with losartan or captopril. J Hypertens. 1997. 15:613–618.
24. Anderson S, Rennke HG, Brenner BM. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest. 1986. 77:1993–2000.

25. Remuzzu A, Perico N, Amuchastegui CS, Malanchini B, Mazerska M, Battaglia C, Bertani T, Remuzzi G. Short and long-term effect of angiotensin II receptor blockade in rats with experimental diabetes. J Am Soc Nephrol. 1993. 4:40–49.
26. Morelli E, Loon N, Meyer TW, Peters W, Myers BD. Effects of converting-enzyme inhibition on barrier function in diabetic glomerulopathy. Diabetes. 1990. 39:76–82.

27. Andersen S, Blouch K, Bialeh J, Deckert M, Parving HH, Myers BD. Glomerular permselectivity in early stages of overt diabetic nephropathy. Kidney Int. 2000. 58:2129–2137.

28. Lee EY, Gil HW, Yang JO, Koh JH, Chung CH, Hong SY. Angiopoietin in diabetic nephropathy. Korean J Nephrol. 2007. 26:311–319.
29. Fujisawa T, Ikegami H, Ono M, Nishino M, Noso S, Kawabata Y, Ogihara T. Combination of half doses of angiotensin type 1 receptor antagonist and angiotensin-converting enzyme inhibitor in diabetic nephropathy. Am J Hypertens. 2005. 18:13–17.

30. Esmatjes E, Fiores L, Inigo P, Lario S, Ruilope LM, Campistol JM. Effect of losartan on TGF-β1 and urinary albumin excretion in patients with type 2 diabetes mellitus and microalbuminuria. Nephrol Dial Transplant. 2001. 161:90–93.

31. Derosa G, Avanzini MA, Geroldi D, Fogari R, Lorini R, Silvestri A, Tinelli C, Rondini G, d'Annuzio G. Matrix metalloproteinase 2 may be a marker of microangiopathy in children and adolescents with type 1 diabetes mellitus. Diabetes Research and Clinical Practice. 2005. 70:119–125.

32. Portik-Dobos V, Anstadt MP, Hutchinson J, Bannan M, Ergul A. Evidence of a matrix metalloproteinase induction/activation system in arterial vasculature and decreased synthesis and activity in diabetes. Diabetes. 2002. 51:3063–3068.
33. Ayo SH, Radnik RA, Garoni JA, Glass WF 2nd, Kreisberg JI. High glucose causes an increase in extracellular matrix proteins in cultured mesangial cells. Am J Pathol. 1990. 136:1339–1348.
34. Kreisberg JI, Garoni JA, Radnik R, Ayo SH. High glucose and TGF beta 1 stimulate fibronectin gene expression through a cAMP response element. Kidney Int. 1994. 46:1019–1024.
35. Rosssing K, Christensen PK, Hansen BV, Cartensen B, Parving HH. Optimal dose of candesartan for renoprotection in type 2 diabetic patients with nephropahty: a double-blind randomized cross-over study. Diabetes Care. 2003. 26:150–155.
36. Ravid M, Savin H, Jutrin I, Bental T, Katz B, Lishner M. Long-term stabilizing effect of angiotensin-converting enzyme inhibition on plasma creatinine and on proteinuria in normotensive type II diabetic patients. Ann Intern Med. 1993. 118:577–581.

37. The EUCLID Study Group. Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. Lancet. 1997. 349:1787–1792.
38. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE study. Lancet. 2000. 355:253–259.
39. Mathiesen ER, Hommel E, Hansen HP, Smidt UM, Parving HH. Randomized controlled trial of long term efficacy of captopril on preservation of kidney function in normotensive patients with insulin dependent diabetes and microalbuminuria. BMJ. 1999. 319:24–25.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download