Abstract
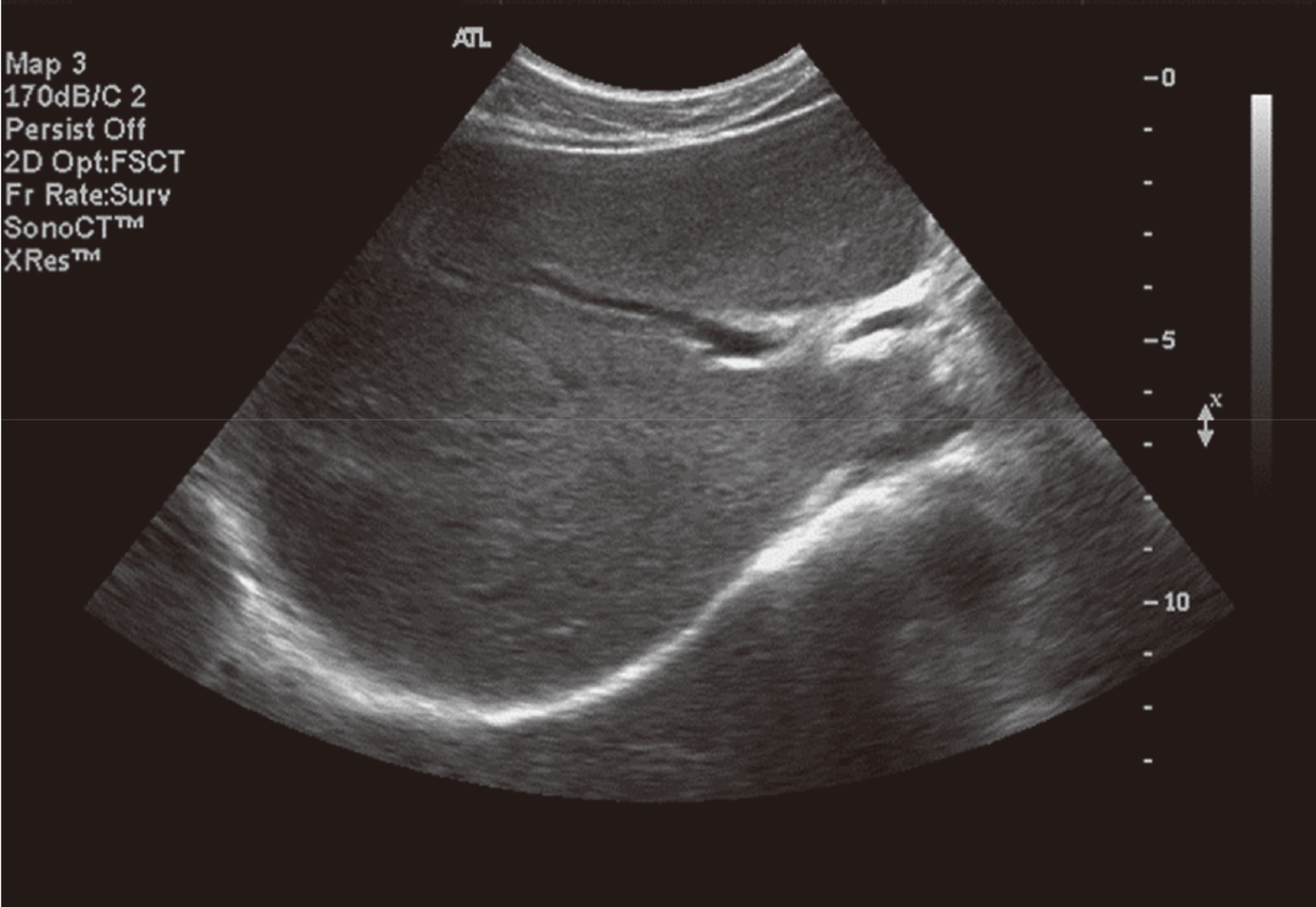
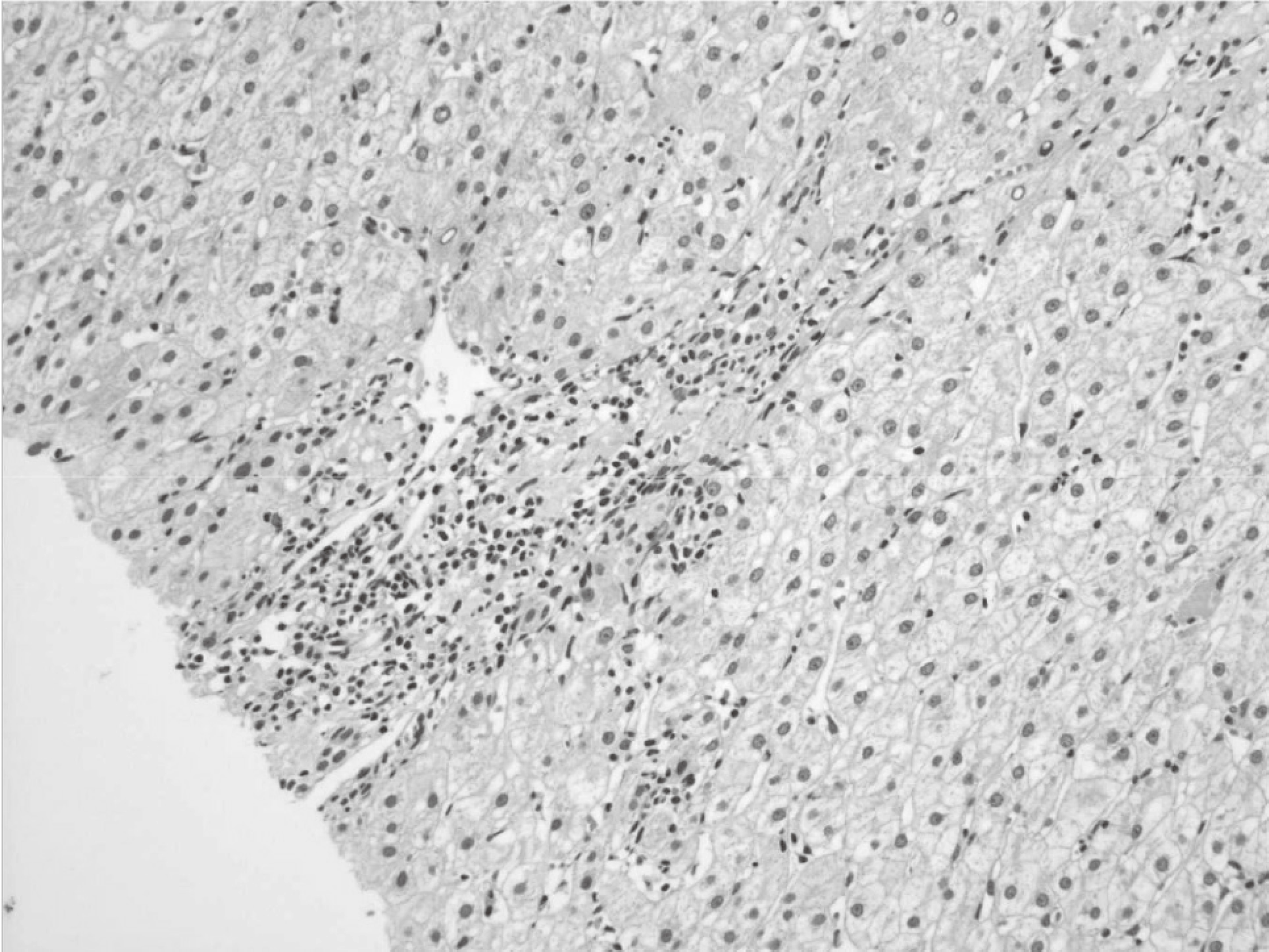
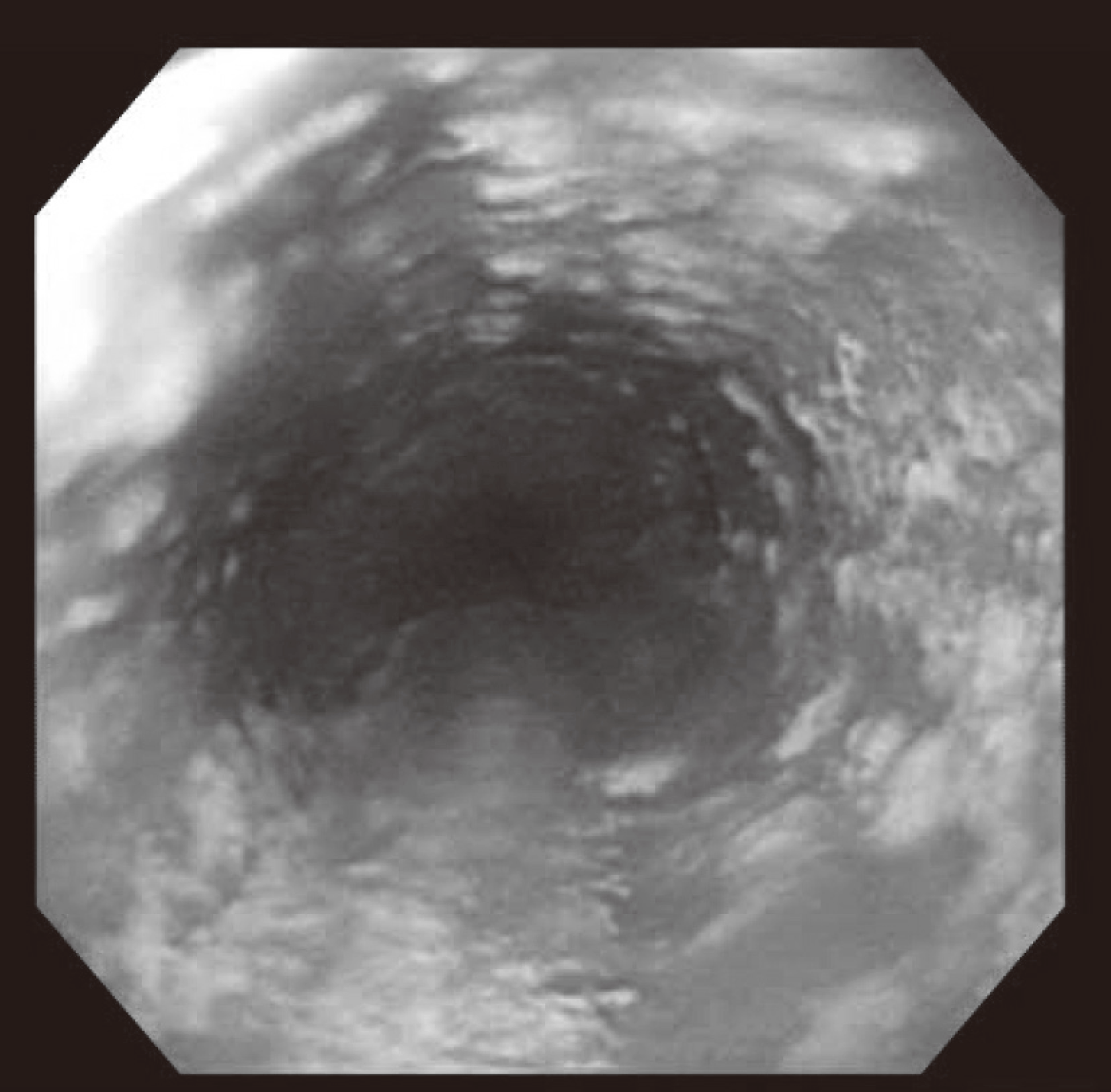
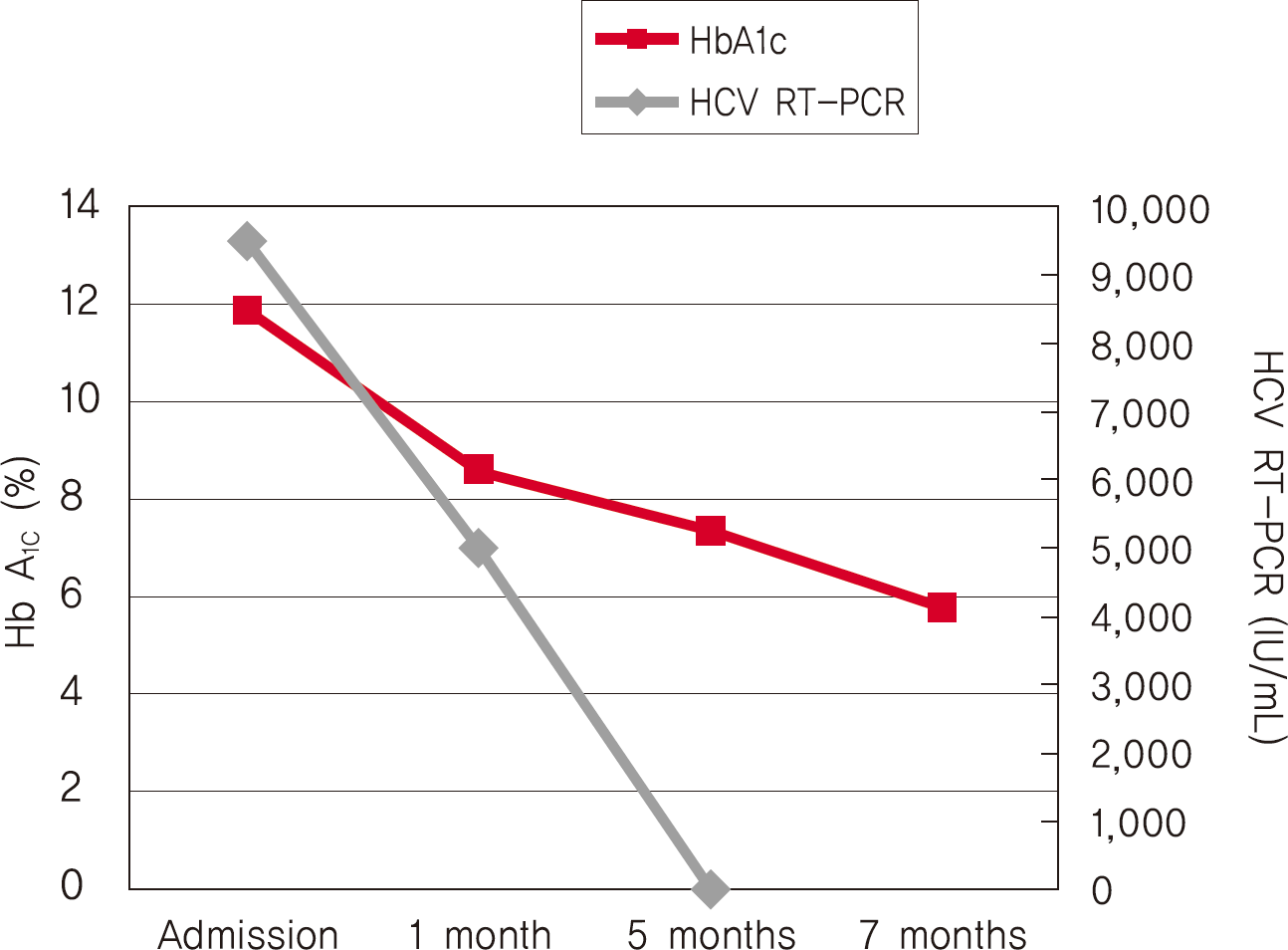
Hepatitis C virus (HCV) mainly affects the liver, but several tissues outside the liver have also been reported to be involved. It has been hypothesized that diabetes could be one of these extrahepatic conditions attributable to HCV infection. The specific mechanisms by which HCV leads to type 2 diabetes are not fully understood, but it seems that an increase in insulin resistance associated with both steatosis and overproduction of proinflammatory cytokines could play a crucial role. We report a patient whose type 2 diabetes that was resolved following interferon-α (IFN-α) therapy for HCV. A 57-year-old man presented with fatigue, polyuria, and polydipsia. He was newly diagnosed as type 2 diabetes and chronic hepatitis C. He was started on subcutaneous insulin and IFN-α. After 24 weeks of treatment with IFN-α, the results of HCV polymerase chain reaction were negative, and his diabetes had resolved. Our case shows a resolution of diabetes after IFN-α therapy for chronic hepatitis C. Although it is unclear whether the resolution of diabetes in this case occurred as an effect of IFN-α or as a result of becoming HCV RNA-negative, our finding suggest roles of IFN-α and HCV infection in the pathogenesis of diabetes.
References
1. Giordanino C, Bugianesi E, Smedile A, Ciancio A, Abate ML, Olivero A, Pellicano R, Cassader M, Gambino R, Bo S, Ciccone G, Rizzetto M, Saracco G. Incidence of type 2 diabetes mellitus and glucose abnormalities in patients with chronic hepatitis C infection by response to treatment: results of a cohort study. Am J Gastroenterol. 2008; 103:2481–7.

2. Poussier A, Lebouvier M, Penfornis A, Di Martino V, Buffier P, Verges B, Hillon P, Petit JM. Specific phenotype associated with diabetes mellitus secondary to chronic hepatitis C infection. Diabet Med. 2008; 25:1237–40.
3. Aytaman A, McFarlane SI. Uncovering glucose abnormalities in people with hepatitis C infection: should oral glucose tolerance test become a standard of care? Am J Gastroenterol. 2008; 103:1941–3.

4. Jeong SH, Kim IH, Kim YS, Kim KA, Lee YJ, Lee JI, Kim CW, Gwak GY, Chung WJ. 2013 Practice guidelines for management of hepatitis C by the Korean Association for the Study of the Liver. KASL. 2013; 2013:76–85.
5. Lee YN, Jeong SW, Lim JH, Ryu YS, Jeon SR, Kim SK, Jang JY, Kim YS, Kim BS, Roh MO. Occurrence of diabetic ketoacidosis and autoimmune thyroiditis in a patient treated with pegylated interferon-alpha 2b and ribavirin for chronic hepatitis C. Korean J Hepatol. 2010; 16:187–91.

6. Tahrani A, Bowler L, Singh P, Coates P. Resolution of diabetes in type 2 diabetic patient treated with IFN-alpha and ribavirin for hepatitis C. Eur J Gastroenterol Hepatol. 2006; 18:291–3.
7. Benegiamo G, Mazzoccoli G, Cappello F, Rappa F, Scibetta N, Oben J, Greco A, Williams R, Andriulli A, Vinciguerra M, Pazienza V. Mutual antagonism between circadian protein period 2 and hepatitis C virus replication in hepatocytes. PLoS One. 2013; 8:e60527.

8. Knobler H, Schattner A. TNF-{alpha}, chronic hepatitis C and diabetes: a novel triad. QJM. 2005; 98:1–6.
9. Zignego AL, Craxì A. Extrahepatic manifestations of hepatitis C virus infection. Clin Liver Dis. 2008; 12:611–36. ix.

10. Kawaguchi T, Ide T, Taniguchi E, Hirano E, Itou M, Sumie S, Nagao Y, Yanagimoto C, Hanada S, Koga H, Sata M. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007; 102:570–6.

11. Cheung O, Sanyal AJ. Hepatitis C infection and nonalcoholic fatty liver disease. Clin Liver Dis. 2008; 12:573–85. viii-ix.

12. Huang JF, Yu ML, Dai CY, Hsieh MY, Hwang SJ, Hsiao PJ, Lee LP, Lin ZY, Chen SC, Hsieh MY, Wang LY, Shin SJ, Chang WY, Chuang WL. Reappraisal of the characteristics of glucose abnormalities in patients with chronic hepatitis C infection. Am J Gastroenterol. 2008; 103:1933–40.

13. Watanabe S, Yaginuma R, Ikejima K, Miyazaki A. Liver diseases and metabolic syndrome. J Gastroenterol. 2008; 43:509–18.

14. Sánchez-Pérez B, Aranda Narváez JM, Santoyo Santoyo J, Fernández-Aguilar JL, Suárez Muñoz MA, González-Sánchez AJ, Pérez Daga JA, Ramírez Plaza CP, Carrasco Campos J, Jiménez Mazure C, Becerra Ortíz R. Influence of immunosuppression and effect of hepatitis C virus on new onset of diabetes mellitus in liver transplant recipients. Transplant Proc. 2008; 40:2994–6.

15. Huang JF, Yu ML, Huang CF, Juo SH, Dai CY, Hsieh MY, Hou NJ, Yeh ML, Hsieh MH, Yang JF, Lin ZY, Chen SC, Shin SJ, Chuang WL. The outcomes of glucose abnormalities in prediabetic chronic hepatitis C patients receiving peginterferon plus ribavirin therapy. Liver Int. 2012; 32:962–9.

16. Zhang W, Rao HY, Feng B, Liu F, Wei L. Effects of interferon-alpha treatment on the incidence of hyperglycemia in chronic hepatitis C patients: a systematic review and metaanalysis. PLoS One. 2012; 7:e39272.

17. Simó R, Lecube A, Genescà J, Esteban JI, Hernández C. Sustained virological response correlates with reduction in the incidence of glucose abnormalities in patients with chronic hepatitis C virus infection. Diabetes Care. 2006; 29:2462–6.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download